February 9, 2017 feature
Battery can be recharged with carbon dioxide
(Phys.org)—Researchers have developed a type of rechargeable battery called a flow cell that can be recharged with a water-based solution containing dissolved carbon dioxide (CO2) emitted from fossil fuel power plants. The device works by taking advantage of the CO2 concentration difference between CO2 emissions and ambient air, which can ultimately be used to generate electricity.
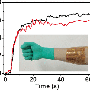
The new flow cell produces an average power density of 0.82 W/m2, which is almost 200 times higher than values obtained using previous similar methods. Although it is not yet clear whether the process could be economically viable on a large scale, the early results appear promising and could be further improved with future research.
The scientists, Taeyong Kim, Bruce E. Logan, and Christopher A. Gorski at The Pennsylvania State University, have published a paper on the new method of CO2-to-electricity conversion in a recent issue of Environmental Science & Technology Letters.
"This work offers an alternative, simpler means to capturing energy from CO2 emissions compared to existing technologies that require expensive catalyst materials and very high temperatures to convert CO2 into useful fuels," Gorski told Phys.org.
While the contrast of gray-white smoke against a blue sky illustrates the adverse environmental impact of burning fossil fuels, the large difference in CO2 concentration between the two gases is also what provides an untapped energy source for generating electricity.
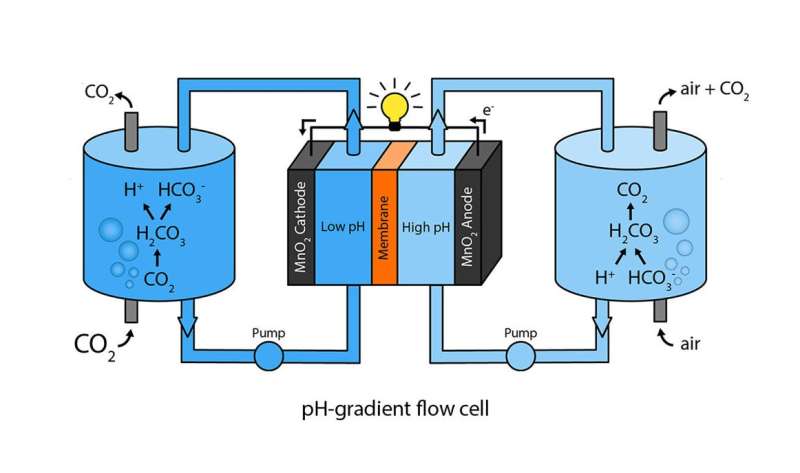
In order to harness the potential energy in this concentration difference, the researchers first dissolved CO2 gas and ambient air in separate containers of an aqueous solution, in a process called sparging. At the end of this process, the CO2-sparged solution forms bicarbonate ions, which give it a lower pH of 7.7 compared to the air-sparged solution, which has a pH of 9.4.
After sparging, the researchers injected each solution into one of two channels in a flow cell, creating a pH gradient in the cell. The flow cell has electrodes on opposite sides of the two channels, along with a semi-porous membrane between the two channels that prevents instant mixing while still allowing ions to pass through. Due to the pH difference between the two solutions, various ions pass through the membrane, creating a voltage difference between the two electrodes and causing electrons to flow along a wire connecting the electrodes.
After the flow cell is discharged, it can be recharged again by switching the channels that the solutions flow through. By switching the solution that flows over each electrode, the charging mechanism is reversed so that the electrons flow in the opposite direction. Tests showed that the cell maintains its performance over 50 cycles of alternating solutions.
The results also showed that, the higher the pH difference between the two channels, the higher the average power density. Although the pH-gradient flow cell achieves a power density that is high compared to similar cells that convert waste CO2 to electricity, it is still much lower than the power densities of fuel cell systems that combine CO2 with other fuels, such as H2.
However, the new flow cell has certain advantages over these other devices, such as its use of inexpensive materials and room-temperature operation. These features make the flow cell attractive for practical applications at existing power plants.
"A system containing numerous identical flow cells would be installed at power plants that combust fossil fuels," Gorski said. "The flue gas emitted from fossil fuel combustion would need to be pre-cooled, then bubbled through a reservoir of water that can be pumped through the flow cells."
In the future, the researchers plan to further improve the flow cell performance.
"We are currently looking to see how the solution conditions can be optimized to maximize the amount of energy produced," Gorski said. "We are also investigating if we can dissolve chemicals in the water that exhibit pH-dependent redox properties, thus allowing us to increase the amount of energy that can be recovered. The latter approach would be analogous to a flow battery, which reduces and oxidizes dissolved chemicals in aqueous solutions, except we are causing them to be reduced and oxidized here by changing the solution pH with CO2."
More information: Taeyoung Kim et al. "A pH-Gradient Flow Cell for Converting Waste CO2 into Electricity." Environmental Science & Technology Letters. DOI: 10.1021/acs.estlett.6b00467
Journal information: Environmental Science & Technology Letters
© 2017 Phys.org