Credit: NIH
Scientists at Van Andel Research Institute and Rockefeller University have successfully described a crucial structure involved in DNA replication, placing another piece in the puzzle of how life propagates.
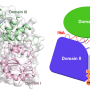
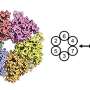
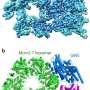
The latest study from long-time collaborators Huilin Li, Ph.D., and Michael O'Donnell, Ph.D., published today in the Proceedings of the National Academy of Sciences, elucidates the interaction between DNA and the eukaryotic enzyme CMG helicase, which opens the DNA double helix like the slider of a zipper and prepares the genetic code for copying.
"Since discovery of the DNA double helix more than 50 years ago, helicase's activity in preparing DNA for replication has been poorly understood," says Li, professor at Van Andel Research Institute. "However, recent advances in microscopy and study design allow us to create accurate images of these enzymes and observe their interactions with DNA for the first time."
More than 40 diseases, including many cancers, anemias and ataxias, in addition to several rare and orphan disorders, trace their origins at least partially to inaccuracy or failures in DNA replication. Results of this study offer a schematic for a core driver behind DNA replication, which Li and O'Donnell hope will eventually help the development of new treatments for these diseases.
"Biologists have learned a great deal about the molecular structure and functions of the enzymes and proteins related to DNA replication," says O'Donnell, professor at Rockefeller University and Howard Hughes Medical Institute investigator. "However, we still have much to discover, and this understanding of helicase activity brings us another step closer."
Findings from the new study reverse a long-held assumption about the orientation of helicase around DNA. Images taken during DNA unwinding demonstrate that helicase's N-tier ring leads the C-tier motor ring and makes first contact with double-stranded DNA. Such orientation is opposite from the currently accepted polarity and has important implications in understanding the mechanism of replication.
Helicase activity has long been recognized as a critical part of DNA replication, itself a fundamental process in the propagation of life. With the publication of this study, scientists have a more complete picture of how most advanced life on Earth proliferates.
This study involved evaluation of CMG helicase purified from the baker's yeast Saccharomyces cerevisiae, an organism commonly used to model higher eukaryotes, including humans.
The structure of the helicase on DNA was derived at Rockefeller University's cryo-electron microscopy (cryo-EM) core facility, leveraging a groundbreaking imaging technology that has revolutionized scientists' ability to visualize and understand the role of fundamental biological processes.
With VARI's recent installation of its own world-class Cryo-EM Core, including the powerful Titan Krios, Li expects the pace of discovery and understanding of these basic biological processes will accelerate in Grand Rapids.
More information: Structure of eukaryotic CMG helicase at a replication fork and implications to replisome architecture and origin initiation, PNAS, www.pnas.org/cgi/doi/10.1073/pnas.1620500114
Journal information: Proceedings of the National Academy of Sciences
Provided by Van Andel Research Institute