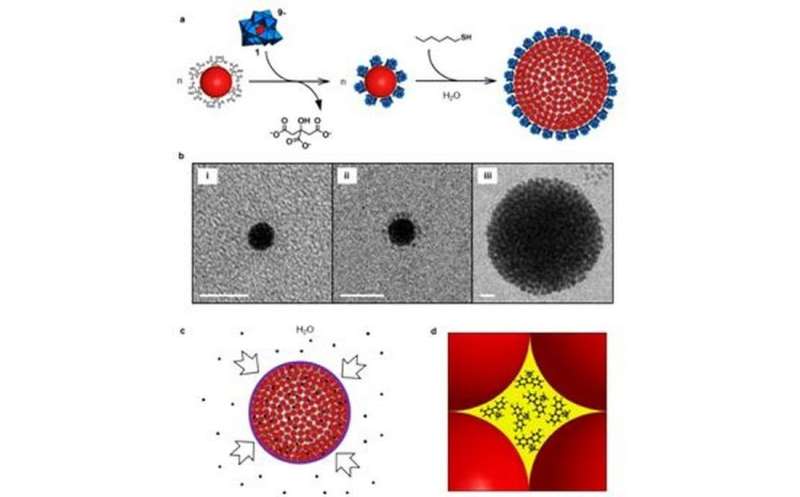
a, Schematic showing how citrate ligands are replaced by AlW11O399- (1), to give 1-protected Au NPs. A reaction with hexanethiol then leads to the formation of colloidal supraspheres. The structure of 1 is shown in polyhedral notation: W(VI)-centred polyhedra are in blue (oxygen atoms are present at all vertices) and the centrally located, four-coordinate Al(III) ion is in red; b, Cryo-TEM images illustrating colloidal suprasphere formation: (i) and (ii) show individual citrate- and 1-protected Au NPs, respectively; (iii) shows a representative intermediate-sized colloidal suprasphere, with a radius of around 45 nm. Scale bar, 10 nm; c, The uptake of bisphenol A (black dots) by the PEG capped supraspheres from aqueous solution; d, Illustration (drawn to scale) of an Oh-symmetry hole hosting approximately 1 nm bisphenol A guests. Credit: Wang et al.
(Phys.org)—Researchers from Ben-Gurion University of the Negev in Israel and École Polytechnique Fédérale de Lausanne in Switzerland have developed porous 200 nm supraspheres from gold nanoparticles whose surface is functionalized with polyoxometalate leaving groups that allows for dispersion in pure water. The hydrophobic effect promotes the spontaneous adsorption of alkyl and alkylaromatic guests.
In their research article in Nature Nanotechnology, Wang et al. demonstrate that their leaving groups are easily displaced by hexane thiol, allowing for the formation of suprasphere colloids in water and uptake of around 2 million hydrophobic guests, a mass-per-volume level that rivals zeolites and metal-organic frameworks. Furthermore, by tailoring the surface of the suprasphere, they were able to chemo-selectively control the uptake of guest molecules.
While there have been several gold nanoparticle suprasphere studies reported in the literature, none of them explore the hydrophobic effect for host-guest interactions. Those interactions largely arise from the formation of aggregates. Supraspheres are gold nanoparticle colloids that are held together by weak interactions between alkylthiolate ligands, which form a hydrophobic monolayer around the nanoparticles. This results in aggregates in pure water.
However, this same hydrophobic effect can be used to promote host-guest interactions. Supraspheres are highly porous compounds that can serve as a reservoir of hydrophobic cavities for non-polar guests in an aqueous environment.
To avoid the problem of uncontrolled precipitation, Wang et al. functionalized the surface of the gold nanoparticles with negatively charged cluster anion, AlW11O399-. They then incrementally added small amounts of hexane thiols (hex-SH) to the solution. Domains of hydrophobic thiol clusters and hydrophilic polyoxometalate clusters formed on the surface of the gold nanoparticles. This eventually leads to the controlled hydrophobic assembly of supraspherical clusters, as evidenced by surface plasmon resonance studies and in situ imaging by cryogenic transmission electron microscopy. These clusters are soluble in water.
Importantly, because there is a linear correlation between the addition of hex-SH and the average hydrodynamic radius, they were able to tailor supracluster formation. Additional studies confirmed supraspheres were formed in water, but that larger supraspheres (approximately 200 nm) were less stable than intermediate-sized ones (approximately 150 nm).
In an effort to keep the larger supraspheres from precipitating out of solution, Wang et al. replaced the remaining polyoxometalates at the cluster-water interface with thiolate-capping ligands. It is the chemical properties of these ligands that allowed for the selective uptake of certain hydrophobic guests over others. They experimented with three caps: a positively changed one, a negatively charged one, and mercapto-polyethylene glycol (PEG-SH).
For their model hydrophobic guest, Wang, et al. used bisphenol A (BPA). Because PEG-S-capped supraspheres are soluble in both water and methylene chloride, they confirmed that guest uptake was due to hydrophobic effects. Adsorption properties were determined with ultraviolet-visible spectroscopy and 1H NMR. They confirmed that each suprasphere hosts the same number of BPA molecules and that the number of guests was around 2 million per suprasphere.
They then explored host-guest properties with other hydrophobic guests. They tested azulene (a dye, TNT (an explosive), RDX (an explosive), alachlor (a common herbicide), para-xylene, and para-dichlorobenzene. At first, they did not observe guest uptake with TNT, which they attributed to kinetic effects. By changing the capping molecules from PEG-SH to a mixture of PEG-SH and hex-SH, they observed the uptake of over 2 million TNT or RDX molecules. This idea of kinetically controlling the uptake of certain guests was used to chemo-selectively adsorb BPA over TNT in a solution containing both molecules.
Analysis of the suprasphere architecture indicated that there was an extensive internal hydrophobic porous system that allowed guests to diffuse throughout the suprasphere structure. The authors described the interior of the suprasphere as a percolated network of hydrophobic holes that can house the more than 2 million guest molecules.
Gold nanoparticle supraspheres are a relatively new area for host-guest chemistry. This research shows how functionalizing the surface of the nanoparticles with a hydrophilic leaving group will allow the formation of water soluble supraspheres that are able to easily and selectively adsorb and release guests.
More information: Yizhan Wang et al. Host–guest chemistry with water-soluble gold nanoparticle supraspheres, Nature Nanotechnology (2016). DOI: 10.1038/nnano.2016.233
Abstract
The uptake of molecular guests, a hallmark of the supramolecular chemistry of cages and containers, has yet to be documented for soluble assemblies of metal nanoparticles. Here we demonstrate that gold nanoparticle-based supraspheres serve as a host for the hydrophobic uptake, transport and subsequent release of over two million organic guests, exceeding by five orders of magnitude the capacities of individual supramolecular cages or containers and rivalling those of zeolites and metal–organic frameworks on a mass-per-volume basis. The supraspheres are prepared in water by adding hexanethiol to polyoxometalate-protected 4 nm gold nanoparticles. Each 200 nm assembly contains hydrophobic cavities between the estimated 27,400 gold building blocks that are connected to one another by nanometre-sized pores. This gives a percolated network that effectively absorbs large numbers of molecules from water, including 600,000, 2,100,000 and 2,600,000 molecules (35, 190 and 234 g l−1) of para-dichorobenzene, bisphenol A and trinitrotoluene, respectively.
Journal information: Nature Nanotechnology
© 2016 Phys.org