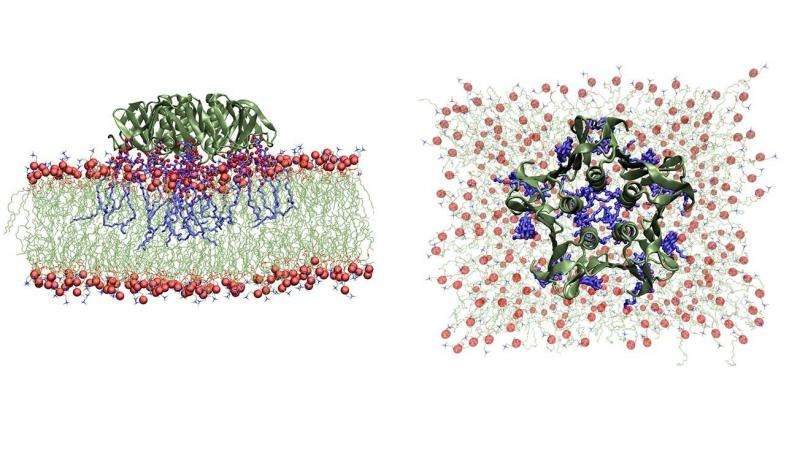
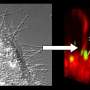
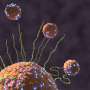
Snapshots from a Molecular Dynamics simulation of a single shigella toxin particle binding to its lipid partners in the vesicle membrane (side and top views). Credit: Julian Shillcock/EPFL
The bacteria that cause the shigella intestinal disease use a toxin that exploits a physical force in the membrane of cells. Though difficult to block, it is possible to fight with nanoparticles exploiting the same force.
An enormous number of diseases are a result of bacterial and virus infections. These pathogens gain entry into cells of the body through several routes. A new study jointly led by EPFL now reports the discovery of a previously unknown infection route used by the bacteria that cause the shigellosis, an intestinal infectious disease characterized by bloody diarrhea. In the newly discovered mechanism, the Shigella bacteria exploits a generic force that is created by the fluctuations of the cell's own plasma membrane. The work is published in ACS Nano.
The study was carried out by John Ipsen at the University of Southern Denmark, Ludger Johannes at the Institut Curie in France and Julian Shillcock at EPFL.
Normally, cells regulate the entry of foreign material very tightly, to prevent invasions from pathogens such as bacteria and viruses. As a result, the invaders have evolved various mechanisms to overcome the barriers and gain entry into cells.
For example, one route involves hijacking the cell's own machinery and tricking it into internalizing the virus or bacterium inside a vesicle that the cell itself makes. This process, which is one of the regular ways cells take in large molecules, is called "endocytosis".
The scientists used various vesicle systems and computer simulations to study a bacterial invasion mechanism that seems to have some unique properties. The mechanism is used, among others, by the bacteria that cause shigella and that produce a small, rigid protein called Shiga toxin.
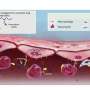
Illustration of a dissipative particle dynamics simulation showing two tightly bound toxins bound together by the Casimir-like force. Credit: Julian Shillcock/EPFL
The study found that Shiga toxin particles bind tightly to certain lipids, or fats, on the membrane surface of the cell to be invaded. They then begin to form clusters on the membrane, which cause the membrane to curve inwards, creating tube-shaped invaginations, through which the toxin particles enter the cell. Once inside, the Shiga toxins modify the cell's genetic mechanism, and infection has begun.
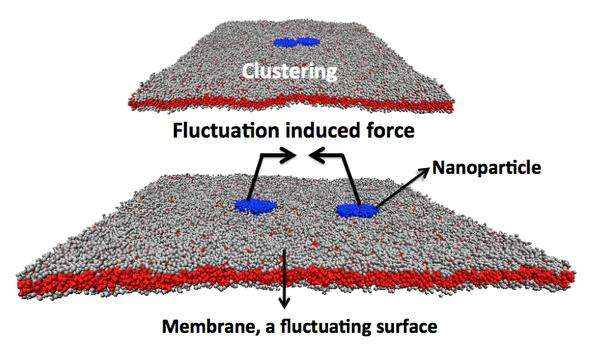
But the major discovery was that the toxin actually exploits a generic, physical force in the cell's membrane to produce the invaginations. This is called the "Casimir force" and was first described as a theoretical force acting between two charged, parallel, conducting surfaces.
In terms of biology, the Casimir force is thought to act between membrane-bound proteins in cells, existing on all fluid biological cell membranes and arising only when the pathogen binds tightly to the membrane surface.
The researchers propose that Shigella bacteria, and other pathogens, have evolved to take advantage of the Casimir force arising from the fluctuating plasma membrane to infect cells. In addition, because the fats that the toxin binds to are used by the cell for its own operations, the Shiga toxin cannot be blocked from entering without disabling or modifying the normal functions of the cell.
Nanoparticles for drug delivery
But because the Casimir force is thought to arise for any tightly bound nanoparticles on the surface of the cell's membrane, there is the potential for producing a novel, nanoparticle-based pathway for drug delivery. First, we would have to bind nanoparticles tightly to the surface of the cell where they will cluster. Second, the nanoparticles must also slightly increase the cell membrane's curvature to exploit the Casimir force and gain entry into the cell. Once inside, they can begin making beneficial, defensive changes in the cell's behavior.
"Where Nature has led in devising a means for pathogens to infect cells, manufactured nanoparticles can follow to treat cellular dysfunction," says Julian Shillcock.
More information: Weria Pezeshkian et al. Mechanism of Shiga Toxin Clustering on Membranes, ACS Nano (2016). DOI: 10.1021/acsnano.6b05706
Journal information: ACS Nano
Provided by Ecole Polytechnique Federale de Lausanne