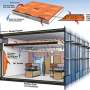
The PCM cube maintains a temperature of 21 degrees Celsius until it is completley melted. Credit: Fraunhofer ICT
When the summer sun burns in the sky, phase change materials (PCM) integrated in building envelopes absorb the heat – it remains cool inside. When it is getting colder outside, the materials give off heat. Several grams of these storage media can protect against overheating and undercooling for a long time. For the first time, researchers have combined insulating characteristics of foams with PCM thermal masses via established procedures of shaping processes. Due to this combination of materials the heat transfer through walls is reduced for hours.
In the evening, it is nice and warm in the living room. However, when you enter the room in the morning it is chilly. It takes time until the heating gets started and the air in the room warms up again. Phase change materials – media made up of salts or organic compounds that store heat – can compensate for such temperature differences. Temperature peaks on hot summer days in indoor areas can also be mitigated.
Insulation and thermal mass
Researchers of the Fraunhofer Institute for Chemical Technology ICT in Pfinztal near Karlsruhe/Germany have combined the traditional advantages of a foamed insulation with the thermic regulating and storing characteristics of PCM within a single component. "The material is able to restore and give off huge amounts of heat within a short time intervall where it changes its temparature while tranforming to another aggregate condition. Via established procedures of shaping processes the PCM were integrated in foamed sheets for the first time. The next step will be to test the long-term resistance of these components," explains Sandra Pappert, a scientist at the ICT. Storage media are already available as microcapsules; they can be stirred into wall paint or plaster. What is special about the new technology: "Instead of a few micrograms, several grams of the phase change materials have been integrated. Therefore the thickness of the wall is not changing by increasing the thermal mass," says Pappert.
The physical principle is known from lakes, which are covered by an ice layer on bitter cold days. Although the air is much colder, the water has a constant temperature of four degrees Celsius for the entire time until the last drop of water in the lake has frozen to ice. The freezing temperatures that the lake absorbs from the air do not cool the water down any further. Rather, they convert the water into ice.
What are phase change materials?
Phase change materials (technical term PCM) are able to absorb, restore and release huge amounts of heat in a small time intervall while changing their aggregate condition depending on their surrounding temperature. This works by storage media, such as salts or organic compounds, changing their aggregate states as soon as heat is added. If, for example, they change from the liquid to the solid state or vice versa, they absorb or release heat.
Provided by Fraunhofer-Gesellschaft