Credit: ACS
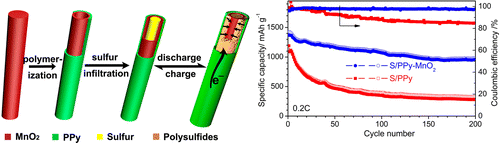
(Phys.org)—A team of researchers at the University of Texas has found that using coaxial nanotubes can improve the performance of lithium-sulfur batteries (Li-S). In their paper published in the journal Nano Letters, the team describes how they used Polypyrrole-MnO2 coaxial nanotubes to overcome obstacles to using Li-S batteries in commercial products.
Prior research has shown that Li-S batteries would offer users of electronics more energy storage—as much as five times that of lithium-ion batteries. They might offer less cost as well, as sulphur is abundant and easily retrieved. Notably, it is also reasonably environmentally safe. But Li-S batteries have suffered from some major problems, the worst being the "shuttle effect"—in which polysulfides move through the cathode and cause problems in the electrolyte. They also move through the electrode, which depletes the sulfur after the battery is charged and recharged just a few times. In this new effort, the researchers have sought to overcome these problems by introducing PPy-MnO2 nanotubes to the design, using them to encapsulate the electrodes. Adding the nanotubes helped to reduce the shuttle effect and also counteracted the problem of low conductivity of sulphur and lithium sulfide, the team reports. It also solved the problem of large changes in volume due to the flexibility of the nanotubes.
The researchers report that testing of their Li-S batteries showed them to have 98.6 Coulombic efficiency—and after cycling the batteries 500 times they found the discharge rate remained above 500 mAh/g which they deemed stable.
The researchers note that while their initial findings with the improved Li-S batteries have been positive, there are still some challenges that must be faced before the batteries can be used in devices—primary among those is "the dendrite problem." This is when branches of lithium grow through the battery separator, causing short circuiting and possibly explosion. The team plans to test the possibility of using other types of electrolytes or using Li2S as part of a cathode that might be paired with anodes made of other materials such as silicon, tin or graphite. They are confident that once a means has been found for stabilizing the anodes, lithium-sulfur batteries will prove useful in a wide range of consumer products.
Journal information: Nano Letters
© 2016 Phys.org