Scientists discover the effect of red light on fluorescent protein
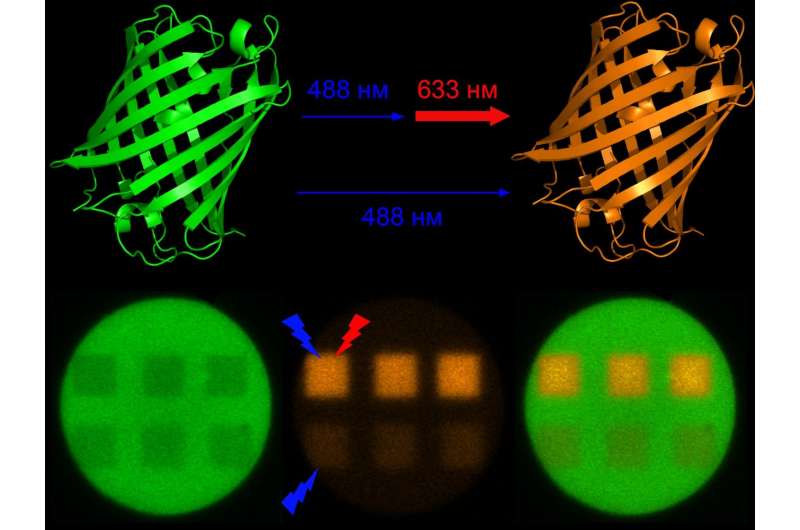
Researchers have developed a new method of switching the Dendra2 fluorescent protein from its green to red form. This phenomenon does not inflict damage to the cells under study, and can be used for a wide range of research. The research results were published in Chemical Сommunications.
Dendra2 is a photo-activated fluorescent protein that was developed 10 years ago by researchers at the M.M. Shemyakin and Yu.A. Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences, based on the Dendra protein from a particular kind of coral, the Dendronephthya sp. octocorals. These proteins are capable of undergoing significant change in their fluorescent properties when irradiated with a certain amount of light of a specific wavelength. Today, they are among the most popular tools for monitoring and tracking proteins, cells and tissues, and are particularly suitable for ultrahigh-resolution fluorescence microscopy.
Studies done on the mechanisms through which this protein are light-activated, in particular switching from green to red form under violet or blue light, had long been considered exhaustive. However, a year ago, researchers discovered that the weak photoactivation of the protein under blue light could be repeatedly strengthened by simultaneous irradiation via laser in the near infrared range (700 to 780 nm). This renders unnecessary the use of violet light, which can harm living tissue. However, the process itself involves using costly infrared lasers.
In the new study, researchers from the Institute of Bioorganic Chemistry of the Russian Academy of Sciences and the Nizhny Novgorod State Medical Academy discovered that even light sources with a substantially shorter wavelength in the red region of the visible spectrum (630-650 nm) are able to induce the same effect.
"During the study, we were able to demonstrate a significant increase in photoactivation efficiency when living cells are simultaneously irradiated with blue and red light under ultrahigh-resolution fluorescence microscopy," notes Alexander Mishin, Ph.D., one of the researchers from the IBCh RAS Laboratory of Biophotonics. "The cheap red lasers that are a standard part of many microscopes have made the new photoactivation method available to a wider range of researchers."
The data obtained from this collaborative research is of particular interest for understanding the photoactivation mechanism itself. As the scientists noted, this process is still riddled with many other unknowns, and even the nature of the unusual intermediate state of the fluorescent protein that absorbs light in a wide spectral range is still a mystery to this day.
Modern science implements several ways of using light to activate these proteins. The most common method involves transforming them from a green to a red fluorescent state. The first such protein was described by Japanese scientists in 2003, who managed to isolate it from madrepore. They named it Kaede, which means 'maple leaf'. However, this is not the only protein capable of changing its fluorescent properties in this way.
More information: N. V. Klementieva et al. Green-to-red primed conversion of Dendra2 using blue and red lasers, Chem. Commun. (2016). DOI: 10.1039/C6CC05599K
Nadya G Gurskaya et al. Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light, Nature Biotechnology (2006). DOI: 10.1038/nbt1191
William P Dempsey et al. In vivo single-cell labeling by confined primed conversion, Nature Methods (2015). DOI: 10.1038/nmeth.3405
Journal information: Nature Biotechnology , Nature Methods