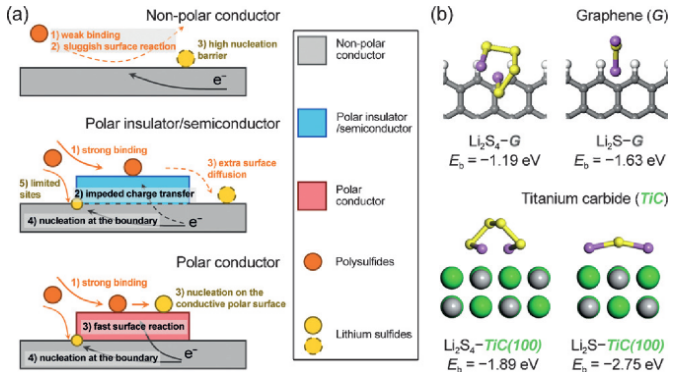
Figure 1. Role of polar conductor in surface reaction and nucleation. (a) Illustration of the working mechanism of the polar conductor as it meets the demand for both adequate binding and charge transfer. (b) First-principle calculations suggesting the higher binding energies of TiC to polysulfides (Li2S4) and Li2S compared to pristine graphene. Credit: Tsinghua University
Developing high-energy, long-life rechargeable batteries is a crucial target to build a sustainable and carbon-neutral society in the future. Most portable electronics, including smart phones and laptops, are powered by rechargeable batteries, and also enable cutting-edge technologies to reduce the emission of carbon dioxide, including electric vehicles and efficient storage of renewable but intermittent energies from solar and wind, which depend on advanced energy storage systems. Alternatives to current lithium-ion battery technologies should possess overwhelming advantage in energy density.
"Lithium-sulfur is one such novel rechargeable battery system. The theoretical gravimetric energy density is 2,600 Wh kg−1, which is about three-fold higher than conventional lithium-ion batteries," said Qiang Zhang, a faculty member at the Department of Chemical Engineering, Tsinghua University. "Additionally, the cathode material, sulfur, is cheap, Earth-abundant and environmentally friendly. All of these desirable attributes make lithium−sulfur batteries promising and also drive us to study lithium−sulfur batteries and to explore the possibility of practical applications."
Lithium−sulfur batteries commonly use liquid electrolytes, in which both sulfur and the final product, lithium sulfide, are solid state, whereas the intermediate polysulfides, which consist of two lithium ions linked by a sulfur chain with different lengths, are highly soluble. These soluble polysulfides consequently diffuse between the sulfur cathode and lithium anode, following a well-known "shuttle" mechanism. Such a mechanism endows lithium−sulfur batteries with a wide range of operating temperature, allowing the employment of lithium−sulfur batteries in harsh environments such as deserts and high latitudes. Some fatal problems, however, also originate from this unique mechanism.
"Owing to the dissolution of polysulfides, the active materials are lost in the electrolyte. More seriously, the shuttling polysulfides diffuse to the lithium anode, igniting the undesirable parasitic reactions between lithium and polysulfides. These reactions, unfortunately, degrade the whole anode structure and lower the battery efficiency," said Hong-Jie Peng, a graduate student at Department of Chemical Engineering, Tsinghua University. "Last but not the least, polysulfides should redeposit onto the cathode. Otherwise, about three quarters of the gross energy cannot be delivered. Highly efficient and controllable redox and deposition of polysulfides are therefore of great importance, which in principle require fundamental understanding of polysulfides' surface behaviors and related key parameters."
"We need a perspective beyond conventional understanding. Catalysis, especially electrocatalysis, is our solution," said Qiang, "Since electrolysis has made a huge success in a broad range of electrochemical reactions such as the oxygen reduction reaction for fuel cells and the water splitting reaction to sustainably produce the hydrogen fuel, why shouldn't it also have a strong impact on lithium-sulfur batteries?"
Analogous to other electrocatalytic reactions, the redox of polysulfides occurs at the electrode surface and involves heterogeneous phases, making it reasonable from a catalysis perspective. "In principle, a heterogeneous catalytic process involves multiple steps. Firstly, the reactant should be adsorbed on a certain surface. Secondly, the surface should impact the chemical bond to allow its transformation. Specifically for the electrocatalysis, the surface must be able to exchange electrons with the adsorbed reactant to drive the redox reactions. Such a coupled adsorption-surface reaction process consequently generates two key aspects to impact the overall electrochemical reaction: how strong the adsorption is, and how fast the surface can transfer the electron," Hong-Jie said.
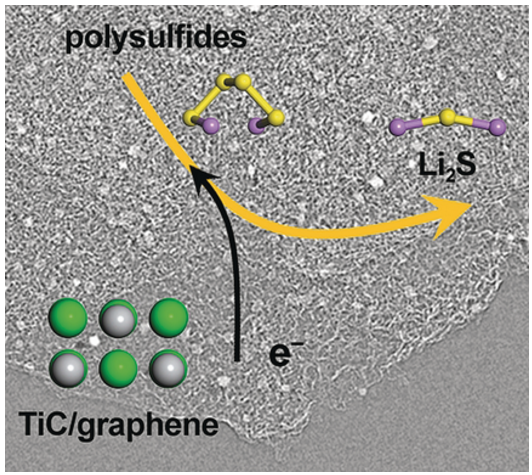
Figure 2 Illustration of The electrochemical reaction kinetics of reversible polysulfide interconversion and Li2S nucleation/precipitation are substantially enhanced on the conductive and polar surface of titanium carbide. Credit: Tsinghua University
In their recent paper published in Angewandte Chemie International Edition on October 10, 2016, they answered the two questions and disclosed how bulk electrical conductivity and surface polarity play complementary roles in the electrocatalysis of electrochemical reactions involving polysulfide species. "We chose three materials with distinct properties to investigate the reaction kinetics on their surfaces. One is graphene, a well-known 'superstar' for its superior electrical conductivity, which is probably the highest among those of all existing materials. But the perfect graphene crystal is non-polar, making it incompatible to the polar polysulfides.", said Ge Zhang, an undergraduate student in Qiang's group and one of the paper's authors. "Another one is titanium oxide, a major component of commercial white paints, having favorable surface polarity. Unfortunately, it is a semiconductor and unable to allow fast charge transfer."
According to their kinetics study, both graphene and titanium oxide exhibited poor activity to catalyze the electrochemical reactions of polysulfide redox, as well as the subsequent conversion to solid lithium sulfides. "The reason is that these two materials only favor one of the two steps in a complete catalytic process. Graphene, highly conductive but nonpolar, is unable to adsorb polysulfides efficiently. By contrast, titanium oxide, poorly conductive but polar, is incapable of transferring electrons generated during the electrochemical reaction," Ge said. "Then we realized that we have to find a material with both sufficient electrical conductivity and surface polarity. Titanium carbide is the 'chosen one.'"
Similar to titanium oxide, titanium carbide has been widely used in manufacturing and machining as a ceramic material. It can be easily produced from titanium oxide. However, the most distinctive property differentiating titanium carbide from titanium oxide is that it offers electrical conductivity 10 orders of magnitude higher, while titanium carbide still has a moderate surface polarity. When conductivity combines with the surface polarity, it makes a big difference. "Using titanium carbides as the electrocatalysts, we are able to improve the reaction kinetics significantly. In a simple experiment to deposit lithium sulfides from polysulfide solutions, the deposition amount on titanium carbide was increased by nearly one fold compared to the pristine carbon electrode or titanium oxide electrode," Hong-Jie said.
This exciting result drove Qiang's group to synthesize highly porous and conductive titanium carbide-based composite cathode materials and to assemble lithium−sulfur batteries with high sulfur loading that approached the practical requirement. Lithium−sulfur batteries employing the as-synthesized titanium carbide-based cathode exhibited reduced internal resistance, enhanced energy efficiency, and prolonged service life.
"We have to say that the battery performance is not the best among all the reported results. But with this work, we strove to understand the role of bulk electrical conductivity and surface polarity in the electrocatalysis of polysulfide conversion," Qiang said. "With such an mechanistic understanding, we are hoping to explore more advanced materials that are both conductive and polar, and to engineer high-performance lithium-sulfur batteries."
More information: Hong-Jie Peng et al. Enhanced Electrochemical Kinetics on Conductive Polar Mediators for Lithium-Sulfur Batteries, Angewandte Chemie International Edition (2016). DOI: 10.1002/anie.201605676
Journal information: Angewandte Chemie International Edition
Provided by Tsinghua University