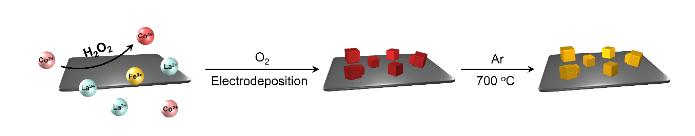
Figure 1. In situ fabrication of the perovskite oxide/nickel foam hybrid through electrodeposition coupled with oxygen reduction reaction and cobalt Fenton process, followed by annealing under Ar protection. Credit: Tsinghua University
Qiang Zhang of the faculty of chemical engineering at Tsinghua University has proposed a novel concept to overcome the intrinsic contradict between oxidative perovskite and the reductive conductive framework through an aqueous oxidation strategy. The monolith exhibited low overpotential and high reactivity for water oxidation. The results have been published in Science Advances.
Water oxidation is has been the bottleneck in many energy devices such as metal-air batteries and water-splitting techniques, calling for new insights in rational design of water oxidation electrocatalysts.
"Development of super-active oxygen evolution electrocatalysts has always been interesting and challenging", said Zhang, "and perovskite oxides are chosen as the focus of our research." The perovskite electrocatalysts calcined at oxidative atmosphere display superb reactivity for water oxidation but suffer from poor electrical conductivity. Most electron pathways contributed by metal current collectors and carbon materials are very easily to be oxidized in most cases.
"We find it is a great challenge to combine the oxidative perovskite and reductive electron pathways", Bo-Quan Li, a graduate student as Department of Chemical Engineering, Tsinghua University, told Phys.Org, "In fact, the perovskite oxides are synthesized by high-temperature annealing under oxygen/air, but conductive frameworks, either carbon or metal, cannot survive in such case." An intrinsic contradiction of in situ hybridization of oxidative perovskite oxides and reductive conductive frameworks is proposed in this system.
In their recent paper published on Science Advances, an effective aqueous peroxidation method was proposed to overcome this contradiction. "Since oxidative annealing is a disaster for carbon or metal, how about separating the oxidation process and annealing process?" This was the first thought that came to Bo-Quan's mind. The group proposed an aqueous pre-oxidation method, as shown in the figure. In this case, the preoxidized perovskite precursor will not need further oxidation during high-temperature annealing and the conductivity of the frameworks can be fully retained.
To verify this concept, the aqueous peroxidation method was introduced to the LaCoFe perovskite oxide/nickel foam catalytic system. H2O2 is used as the intermediate oxidant for peroxidation, and two-electron oxygen evolution is an efficient method to provide in situ H2O2 from O2 in aqueous solution. H2O2 then oxidized Co2+ into Co3+, described as the cobalt Fenton process, which is the core reaction of the peroxidation strategy. Meanwhile, the electrodeposition deposition reaction was applied to generate OH- and stabilize the preoxidized Co3+ by coprecipitation along with La3+ and Fe3+, resulting in a perovskite precursor in situ hybridized with nickel foam. Followed by inert annealing under Ar protection, the in situ hybrid composite of oxidative perovskite and reductive conductive frameworks was fabricated.
The as-synthesized electrocatalyst exhibits desirable morphology and electrocatalytic performance. "Perovskite oxides are nano-sized and uniformly distributed on the surface of nickel foam, and that is exactly what we want," said Bo-Quan, "the perovskite phase is well-crystalized and fully oxidized with cobalt in its high oxidation state. The OER performance is satisfied with an overpotential required at 10 mA cm-2 to be 350 mV and being stable for more than 10000 s with no obvious current density decrease at a constant potential. All the evidence indicates the success of our peroxidation strategy."
The results inaugurate an effective method to integrate the oxidative active phase with a reductive framework. "To our knowledge, oxidative and reductive phases cannot coexist. Yet we chemists overcome such contradictions and make things which seem impossible happen. We believe that our peroxidation method not only is a rational strategy for development of OER electrocatalysts, but also opens our minds to redefine chemistry of the future."
More information: B.-Q. Li et al. An aqueous preoxidation method for monolithic perovskite electrocatalysts with enhanced water oxidation performance, Science Advances (2016). DOI: 10.1126/sciadv.1600495
Journal information: Science Advances
Provided by Tsinghua University