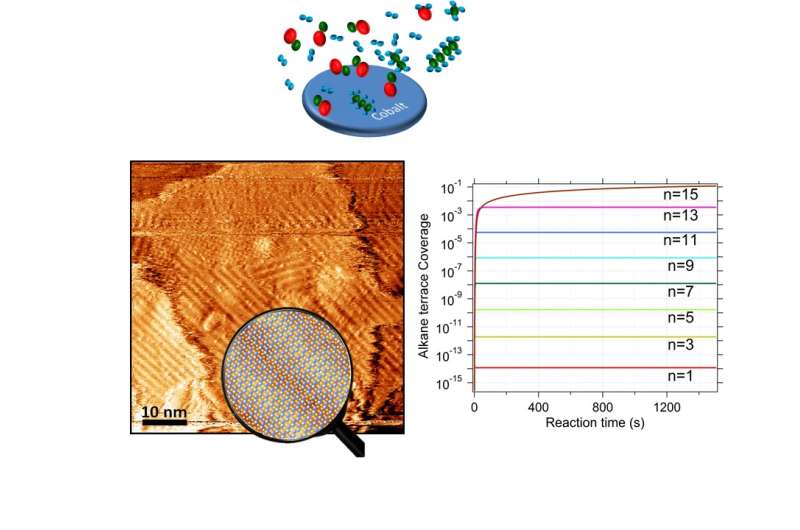
Top: Artist impression of the reactants carbon monoxide and hydrogen and the produced hydrocarbon molecules of different lengths on the cobalt catalyst (carbon atoms are represented in green, oxygen atoms in red and hydrogen atoms in blue). Bottom left: Topographic image of a region (3840 nm2) of the surface of a cobalt catalyst during reaction, obtained with a scanning tunneling microscope. The height in the image is represented in a color scale where the darker colors are lower than the lighter ones, and the total height is 1.4 nm. The image was taken after 40 minutes of reaction at 221 °C and a pressure of 4 atmospheres in a mixture of the gases carbon monoxide, hydrogen and argon in the ratio 1:2:2 respectively. The cobalt surface is covered by a stripped pattern which results from the organization of the molecules produced during the reaction, that align next to each other in a regular pattern, similar to cars in a parking lot. The magnifying glass shows an impression of how the molecules are organized within the stripes. Bottom right: Graphic representation of the concentration of the molecules produced during the reaction on the cobalt catalyst surface as a function of the reaction time, depending on their length. All lengths are produced during the reaction, but the shorter molecules are very volatile and leave the surface fast. Longer molecules reach higher concentrations on the surface of the catalysts. The molecules with 15 carbon atoms are the first ones that reach a concentration on the surface which is high enough to organize themselves in a striped regular pattern. Credit: Leiden Institute of Physics
Synthetic fuel is cleaner than natural oil, but its production process is inefficient. Now, for the first time, physicists have directly observed the molecules produced in the chemical process. This paves the way for designing more efficient catalysts.
To date, natural oil still serves as our primary source of fuel, even though a much cleaner alternative exists in the form of synthetic fuel. This contains much less sulfur and doesn't require oil as the starting material. At the moment, only 5 percent of the world's production of diesel fuel makes use of this process because it is cheaper for companies to use more polluting substances. If researchers gain a better understanding of the production process of synthetic fuel, the balance could tip the other way.
Now, physicists from Leiden University have observed for the first time how this chemical process unfolds in the early stages. They already knew that the necessary chemical reaction between carbon monoxide and hydrogen takes place on the surface of small cobalt particles. These serve as the catalyst for the reaction. It is, however, very difficult to verify the exact working mechanism in experiments. Researchers have to deal with pressures of several atmospheres and temperatures of several hundred degrees Celsius. These are far from ideal circumstances for observing molecules. Group leader Joost Frenken and his team developed a special type of Scanning Tunneling Microscope—a so-called Reactor-STM—to bypass this problem.
To their surprise, they observed that in the first stages of the process, the surface covers itself up progressively in a single layer of hydrocarbon molecules with a highly ordered regular pattern. The molecules accumulate on the cobalt surface with the same length. The Leiden physicists were able to explain these findings with a simple theory: the catalyst constructs the molecules step-by-step at the atomic steps on the cobalt surface. Most molecules spend some time on the surface and then evaporate, but the longer ones adhere more strongly and fill the surface. The most efficient way to do that is to fill the surface with a regular pattern, similar to cars in a parking lot.
Currently, catalysts are being developed mostly by trial and error. With the new discovery, first author Violeta Navarro and her colleagues pave the way for future generations of genuine 'designer' catalysts, with fully optimized efficiency and selectivity for the desired products. Frenken says, "The ultimate goal is that of true "designer catalysts". We're absolutely not there yet, but understanding the early stages of synthetic fuel production forms an essential component of unraveling the entire, complex reaction mechanism. We have introduced a new way of looking at an active catalyst with the ultimate resolution."
More information: Violeta Navarro et al. In situ observation of self-assembled hydrocarbon Fischer–Tropsch products on a cobalt catalyst, Nature Chemistry (2016). DOI: 10.1038/nchem.2613
Journal information: Nature Chemistry
Provided by Leiden Institute of Physics