August 18, 2016 report
A practical synthesis for benzazetidine compounds
(Phys.org)—A key chemical component to antibiotics, such as penicillin is the beta-lactam, a four-membered amide ring that is fused to another heterocycle. Researchers in drug design would like to explore compounds using a similar structure. One avenue may be found in the four-member azetidine ring. Similar to the beta-lactam ring, it is a four-membered amine heterocycle. However, less progress has been made for benzazetidines, an azetidine fused to a benzene ring, due to the significant ring strain in these four-membered scaffolds.
A group of researchers from Nankai University in China, the University of Pittsburgh, and The Pennsylvania State University have demonstrated, for the first time, a synthetic mechanism for N-unsubstituted benzazetidines that is high yielding and practical. Their synthetic strategy can be used to make a variety of benzazetidine-based compounds for possible drug design exploration. Their work appears in Nature Chemistry.
Researchers have made headway in nitrogen-based heterocycle chemistry using a palladium-catalyzed intramolecular dehydrogenative C-H amination (IDCA) reaction. In this reaction, the oxidized palladium catalyst coordinates to the target carbon and the amine. Ideally, this would result in a reductive elimination reaction in which the palladium is reduced back to its original oxidation state and the dehydrogenated carbon and amine would form a ring-closing bond.
However, in practice, this reaction is difficult to accomplish in high yields. Four-membered rings are hindered due to ring strain. This leads to side products that are more thermodynamically favored than forming the four-membered ring.
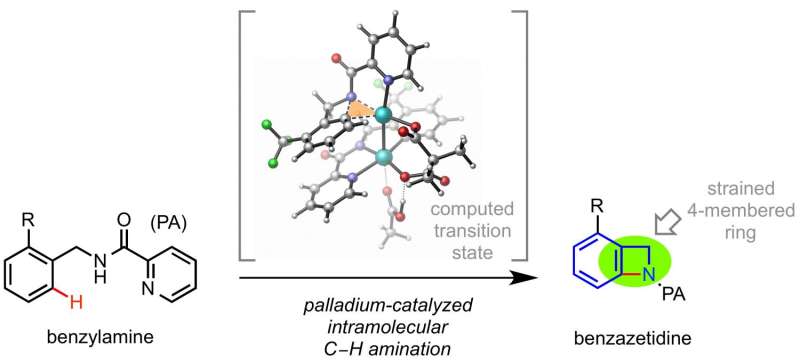
Gang He, Gang Lu, Zhengwei Guo, Peng Liu, and Gong Chen have devised a synthetic scheme that results in the desired benzazetidine using N-benzyl-picolinamide (PAs) as the reactant and Pd(OAc)2 as the catalyst. Key to the success of their synthetic scheme is rigid ligand structures both from the N-benzyl-picolinamide and the oxidant.
They developed the phenyl-iodonium dimethylmalonate (PhI(DMM)) as their oxidant after seeing that PhI(OAc)2, which is known to promote C-H acetoxylation using similar starting materials, formed a small amount of their target benzazetidine. However, the C-N ring closing reaction is thermodynamically unfavored compared to forming a C-OAc bond. After looking at computational studies to see how they could prevent the carbon-oxygen bond from forming, they decided to tether the oxygen on the carbonyl of the acetate to prevent it from reacting with the target carbon atom for the reductive elimination. After trying several tethers, they landed upon PhI(DMM), which proved to enhance the yield of the desired benzazetidine (48%).
The next step was to see if this reaction was generalizable by changing the R group on the N-benzyl picolinamide reactant. Even in cases where the benzazetidine product could be formed using PhI(OAc)2, He, et al. saw better yields with PhI(DMM). Furthermore, their reaction worked with a variety of functional groups, although, interestingly, did not work for unsubstituted benzylamine. An additional advantage of their scheme is that the PA protecting group was easily removed with sodium hydroxide in methanol, THF, and water at room temperature.
He, et al. conducted mechanistic studies in hopes of understanding the reaction better so that it may be optimized for later research,. They found that the transition state involves a bimetallic Pd(III)/Pd(III) complex instead of a monomeric Pd(IV) compound. It is this bimetallic complex with the PhI(DMM) tether that promotes the desired reductive elimination pathway and blocks the C-OAc bond from forming.
This research opens the door to the possibility of finding pharmaceuticals that involve a benzazetidine. This synthetic mechanism is versatile for various functional groups and, using the PhI(DMM) oxidizing agent, produces product yields that make this reaction pathway significantly more practical than the limited reaction mechanisms that were previously used to make benzazetidines.
More information: Gang He et al. Benzazetidine synthesis via palladium-catalysed intramolecular C−H amination, Nature Chemistry (2016). DOI: 10.1038/nchem.2585
Abstract
Small-sized N-heterocycles are important structures in organic synthesis and medicinal chemistry. Palladium-catalysed intramolecular aminations of the C−H bonds of unfunctionalized amine precursors have recently emerged as an attractive new method for N-heterocycle synthesis. However, the way to control the reactivity of high-valent Pd intermediates to form the desired C−N cyclized products selectively remains poorly addressed. Herein we report a strategy to control the reductive elimination (RE) pathways in high-valent Pd catalysis and apply this strategy to achieve the synthesis of highly strained four-membered benzazetidines via the Pd-catalysed intramolecular C−H amination of N-benzyl picolinamides. These reactions represent the first practical synthetic method for benzazetidines and enable access to a range of complex benzazetidines from easily obtainable starting materials. The use of a newly designed phenyliodonium dimethylmalonate reagent is critical, as oxidation of Pd(II) palladacycles with this reagent favours a kinetically controlled C−N RE pathway to give strained ring-closed products.
Journal information: Nature Chemistry
© 2016 Phys.org