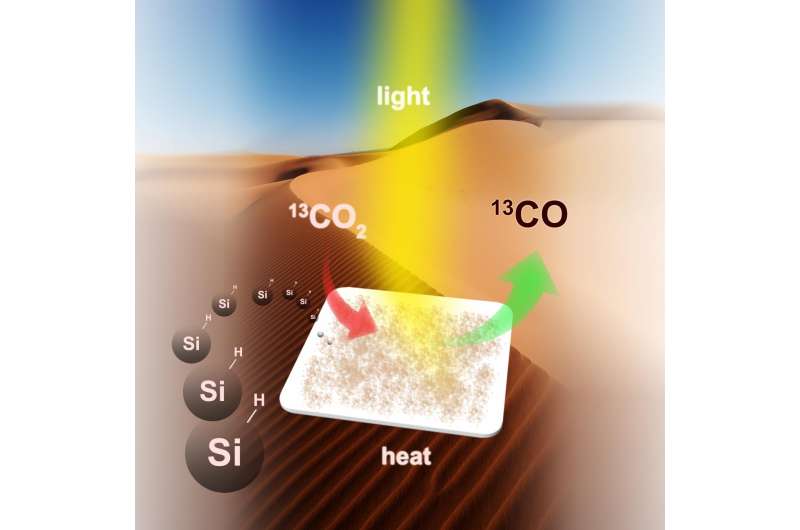
Converting greenhouse gas emissions into energy-rich fuel using nano silicon (Si) in a carbon-neutral carbon-cycle is illustrated. Credit: Chenxi Qian
Every year, humans advance climate change and global warming - and quite likely our own eventual extinction - by injecting about 30 billion tonnes of carbon dioxide into the atmosphere.
A team of scientists from the University of Toronto (U of T) believes they've found a way to convert all these emissions into energy-rich fuel in a carbon-neutral cycle that uses a very abundant natural resource: silicon. Silicon, readily available in sand, is the seventh most-abundant element in the universe and the second most-abundant element in the earth's crust.
The idea of converting carbon dioxide emissions to energy isn't new: there's been a global race to discover a material that can efficiently convert sunlight, carbon dioxide and water or hydrogen to fuel for decades. However, the chemical stability of carbon dioxide has made it difficult to find a practical solution.
"A chemistry solution to climate change requires a material that is a highly active and selective catalyst to enable the conversion of carbon dioxide to fuel. It also needs to be made of elements that are low cost, non-toxic and readily available," said Geoffrey Ozin, a chemistry professor in U of T's Faculty of Arts & Science, the Canada Research Chair in Materials Chemistry and lead of U of T's Solar Fuels Research Cluster.
In an article in Nature Communications published August 23, Ozin and colleagues report silicon nanocrystals that meet all the criteria. The hydride-terminated silicon nanocrystals - nanostructured hydrides for short - have an average diameter of 3.5 nanometres and feature a surface area and optical absorption strength sufficient to efficiently harvest the near-infrared, visible and ultraviolet wavelengths of light from the sun together with a powerful chemical-reducing agent on the surface that efficiently and selectively converts gaseous carbon dioxide to gaseous carbon monoxide.
The potential result: energy without harmful emissions.
"Making use of the reducing power of nanostructured hydrides is a conceptually distinct and commercially interesting strategy for making fuels directly from sunlight," said Ozin.
The U of T Solar Fuels Research Cluster is working to find ways and means to increase the activity, enhance the scale, and boost the rate of production. Their goal is a laboratory demonstration unit and, if successful, a pilot solar refinery.
More information: Wei Sun et al, Heterogeneous reduction of carbon dioxide by hydride-terminated silicon nanocrystals, Nature Communications (2016). DOI: 10.1038/ncomms12553
Journal information: Nature Communications
Provided by University of Toronto