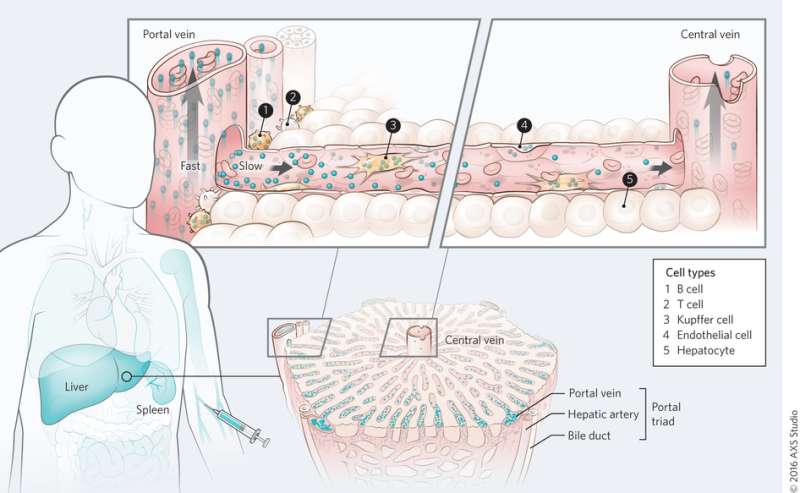
Mechanism of nanomaterial transport in the liver. Credit: (c) Kim M. Tsoi, et al. Nature Materials (2016) doi:10.1038/nmat4718
(Phys.org)—One of the biggest challenges to realizing the potential of targeted therapies is keeping nanomaterials from accumulating in the liver or spleen. The liver and spleen are part of the mononuclear phagocyte system. Its job is to filter toxins from the blood stream. Unfortunately, in doing its job, it is also preventing nanotherapies from reaching their target.
To overcome this obstacle a group of researchers from several institutions in Toronto have conducted organ-level and sub-organ level computational, in vitro, and in vivo studies using quantum dots, gold nanoparticles, and silica nanoparticles to better understand the mononuclear phagocyte system and the mechanism by which nanoparticles are sequestered. They found that blood flow rate, cellular phenotype, and physical position in the liver all play a role in nanoparticle uptake. They suggest that future work ought to involve not only nanoparticle design, but some kind of liver pre-conditioning. Their work appears in Nature Materials.
Nanoparticles can be functionalized in such a way that the particle targets a particular cell type. This holds great promise for cancer and other targeted therapies. However, when nanotherapies are tested in the body, the nanoparticle is cleared from the bloodstream via the mononuclear phagocyte system (MPS). This holds true for all types of nanoparticles.
Tsoi, et al. conducted whole-organ and sub-organ analyses to better understand how the MPS system sequesters nanoparticles. For their experiments, they focused on non-degradable "hard" nanoparticles: quantum dots, gold nanoparticles, and silica nanoparticles.
On the whole organ level, Tsoi, et al. found that quantum dots are first cleared by the cells near the portal triad and that there is a clearance gradient through the liver sinusoid during the first pass. Blood flows into the liver through the portal triad and out through the central vein. This was also observed with gold nanoparticles irrespective of surface functionalization, although protein adsorption seemed to play a role in nanoparticle uptake.
The next area of investigation is whether blood flow rate plays a role in nanoparticle sequestration. Blood flow slows down once it hits the liver (from 10-100 cm s-1 to 200-800 μm s-1). Tsoi, et al. developed a mathematical model to describe blood flow within the liver and the probability of nanoparticle sequestration. They then compared their computational results to the results of cytometry studies with the rats that were treated with quantum dots in the test for nanoparticle accumulation. Notably, while advection is the dominant influence on blood flow in the body, diffusion is the dominant influence in the liver. They found that the liver was 102 to 103 times more likely to sequester nanomaterials and that particle size played a role – the larger the particle, the more likely it was absorbed by the liver.
At the sub-organ level, Tsoi, et al. looked at which cell types play the biggest role in nanoparticle uptake. Studies to determine cell uptake of quantum dots showed that Kupffer cells adsorbed the largest volume of quantum dots, as expected. However, what was surprising was the number of particles internalized by B cells. B cells seem to play a much bigger role in nanoparticle uptake than was once thought, although Kupffer cells are still the key cells in removing nanoparticles. Other cell types, including endothelial cells, also played a role in removing nanoparticles.
Next, Tsoi, et al. tested whether organ architecture affects nanoparticle uptake in the liver by studying the sequestration process in the spleen. They found that of the nanoparticles that were removed by the spleen, almost all of them were located in the red pulp region. This is where blood flow decreases compared to the flow throughout the body. While some nanoparticles resided in the spleen, spleen macrophages internalized fewer nanoparticles than Kupffer cells within the liver. This was confirmed with comparative in vitro and in vivo studies, and demonstrates that organ architecture cell-type play a role in nanoparticle uptake.
This research provides important insights in how to overcome nanoparticle uptake by the MPS. Typically researchers focus on the nanoparticle design, but this study suggests that bodily environment plays an important role in nanoparticle sequestration. The authors suggest manipulating the host environment as a complementary strategy to nanoparticle optimization. Preliminary tests show that two possible avenues are changing the blood flow rate through the liver and changing the phenotype of certain cells so they are not prone to nanomaterial uptake.
More information: Kim M. Tsoi et al. Mechanism of hard-nanomaterial clearance by the liver, Nature Materials (2016). DOI: 10.1038/nmat4718
Abstract
The liver and spleen are major biological barriers to translating nanomedicines because they sequester the majority of administered nanomaterials and prevent delivery to diseased tissue. Here we examined the blood clearance mechanism of administered hard nanomaterials in relation to blood flow dynamics, organ microarchitecture and cellular phenotype. We found that nanomaterial velocity reduces 1,000-fold as they enter and traverse the liver, leading to 7.5 times more nanomaterial interaction with hepatic cells relative to peripheral cells. In the liver, Kupffer cells (84.8 ± 6.4%), hepatic B cells (81.5 ± 9.3%) and liver sinusoidal endothelial cells (64.6 ± 13.7%) interacted with administered PEGylated quantum dots, but splenic macrophages took up less material (25.4 ± 10.1%) due to differences in phenotype. The uptake patterns were similar for two other nanomaterial types and five different surface chemistries. Potential new strategies to overcome off-target nanomaterial accumulation may involve manipulating intra-organ flow dynamics and modulating the cellular phenotype to alter hepatic cell interactions.
Journal information: Nature Materials
© 2016 Phys.org