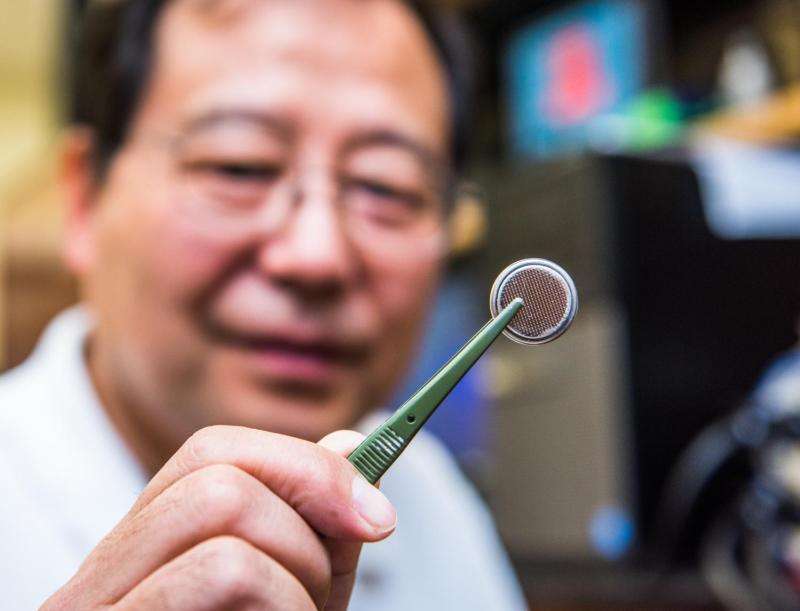
Jun Li, professor of chemistry at Kansas State University, holds a patented lithium-ion battery anode, which he invented with Steven Klankowski, doctoral graduate, and Ronald A. Rojeski, of Catalyst Power Technologies Inc.
Inventors at Kansas State University and Catalyst Power Technologies Inc. are paving the way for the future with energy-efficient batteries for sensors, portable devices and electric cars.
Jun Li, professor of chemistry; Steven Klankowski, May 2015 chemistry doctoral graduate, La Crescent, Minnesota; and Ronald A. Rojeski, Catalyst Power Technologies Inc., Santa Clara, California, received a patent for their lithium-ion battery anode, including a core-shell heterostructure of silicon-coated vertically aligned carbon nanofibers.
The inventors are adjusting structure, composition and other factors to store more energy per gram, thereby reducing the battery's weight, making it run longer and improving the power output.
The invention has been featured in about 30 presentations and publications, and was on the cover of the Journal of Materials Chemistry A, a scientific journal published by the Royal Society of Chemistry. The patent was issued to the Kansas State University Research Foundation, a nonprofit corporation responsible for managing technology transfer activities at the university.
This invention uses silicon rather than graphite or other carbonaceous materials—common anodes in current commercial batteries—so it provides higher capacity limits, shorter charging periods and significantly longer battery lifetimes.
"One key component of the electric car is the battery, so we ask, 'After it is charged, how many miles can you drive?'" Li said. "We also examine how long it takes to charge the battery after it's used."
The price point is pivotal. For example, 70 percent of the cost of Tesla's newest version, the Model 3, goes to the battery, according to Li. Unveiled July 29, the new model is scheduled to hit the market in late 2017 with a projected cost of $35,000.
"To lower the cost is one thing, but just as important is if you can create a better battery with a similar cost," Li said. "If the batteries are priced similarly, but this battery can run longer and provide more energy, it's equivalent to the advantage of paying less."
In addition to all-electric cars, the batteries could be used in hybrid vehicles, portable electronics, power tools and military and outer space applications. National Science Foundation, NASA and the state of Kansas have provided funding for the research. Catalyst Power Technologies Inc. is pushing this technology to the market.
"After 28 years working with these nanomaterials, it is highly rewarding to find an application with such influence on the future of transportation and electronics," Li said. "I am excited to see our work in the lab transfer to industry products."
More information: Jun Li et al. Pd-Catalyzed oxidative isomerization of propargylic acetates: highly efficient access to α-acetoxyenones via alkenyl Csp–O bond-forming reductive elimination from Pd, Chem. Commun. (2016). DOI: 10.1039/C6CC04463H
Journal information: Journal of Materials Chemistry A
Provided by Kansas State University