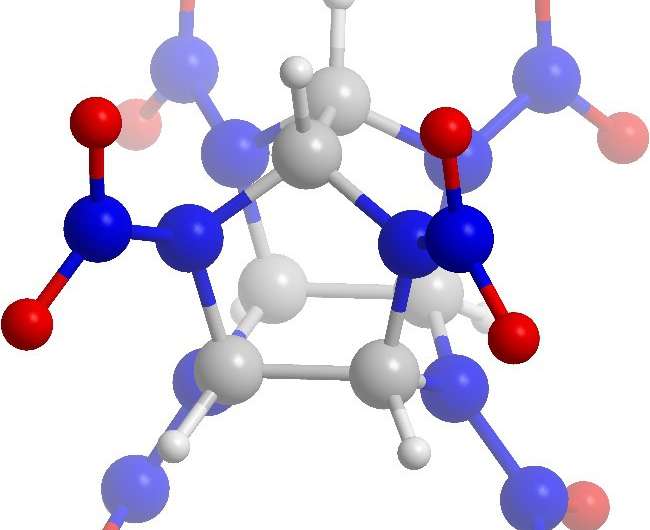
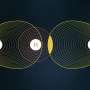
Figure 1. CL-20 molecule. Credit: National Research Nuclear University
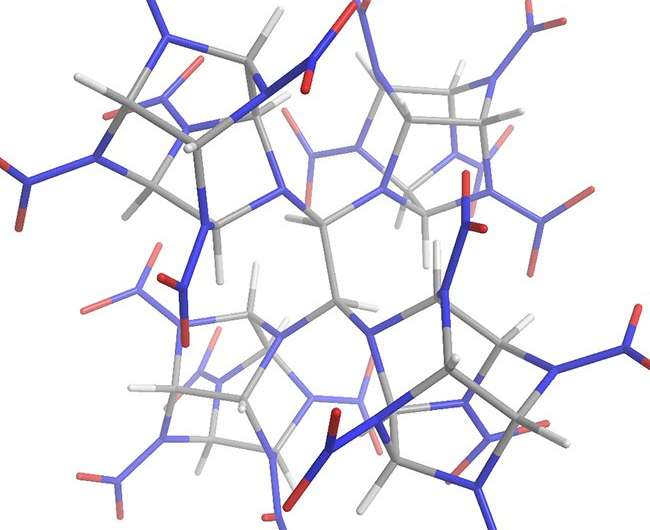
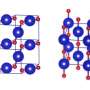
MEPhI researchers have shown that CL-20 molecules can covalently bind with the help of "molecular bridges," making dimers and tetramers (Fig.2) stable at liquid nitrogen boiling temperature.
Researchers plan to make a detailed computer-aided simulation using quantum physics and the chemistry of potential covalent crystal structures on the basis of CL-20 molecules and consider the opportunity to raise the stability of these crystals using "molecular bridges. If successful, it will be possible to predict a consistent set of physical-chemical characteristics of new materials.
Chemical compound CL-20, first synthesized in 1987, is of special fundamental and applied interest. The technological process of getting CL-20 was established in the 1990s and is now considered a next-generation fuel component. However, there are impediments to widespread usage of CL-20— in particular, its high cost and the complexity of production with the necessary degree of purity.
CL-20 is an isolated molecule with a carbon-nitrogen construction formed of two pentatomic and one hexatomic ring with six functional nitrogroups connected with carbon-carbon bonds (Fig.1).
Although CL-20 is metastable, its high stability is proved by van der Waals crystals. Currently, five polymorphic variations of these crystals are known, which are different in molecular organization and mutual orientation of nitrogroups, but only four of them are stable in standard conditions. Despite active research of CL-20, as well as solid materials based on them, there has been no data about experimental synthesis or theoretical analysis of covalent crystals from CL-20 compounds. It is well-known that other nanostructures with a "strained" carcass—fullerene, for example—are able to create not only molecular, but also covalent complexes.
Figure 2. Covalent crystal CL-20 fragment (tetramer). Credit: National Research Nuclear University
That means there is a possibility of getting covalent crystals of CL-20, which can guarantee a closer packing of these molecular systems, raise the kinetic stability of crystals, improve energy efficiency and, most likely, reduce costs.
Provided by National Research Nuclear University