June 7, 2016 report
Using the 'deuterium switch' to understand how receptors work
(Phys.org)—The market value for deuterated drugs has recently been estimated at over a billion dollars. Such drugs are simply molecules in which one or more hydrogen atoms are replaced with deuterium. While these kinds of manipulations are known to work wonders as far as breathing new life into aging patents, the overall therapeutic value of this medical manna can be contentious. A recent paper published in PLoS ONE seeks to explain the 'quantum nature of drug-receptor interactions' under deuteration using a combined experimental and computational approach. Although a tall order, a more comprehensive and predictive theory of receptor interactions is sorely needed. Perhaps a theory in which the molecular character of drug effects are written less into the receptor and more into the drug itself.
The authors measured changes in the binding affinities of histamine receptor ligands after they replaced the normal buffer solution with D20 (deuterium oxide). In contrast to other kinds of studies in which the ligands themselves had deuterium permanently bound to carbon atoms, a heavy water solution would deuterate the ligand at exchangeable N-H and O-H protons. This trick directly targets the hydrogen bonds that presumably control ligand-receptor interactions and associated ligand-water interactions.
There is no shortage of ways in which an extra neutron perturbs the life of a molecule. A two-fold mass gain decreases bond length and increases bond strength. This ultimately changes a number of physical and chemical properties, including molar volume, polarity, electron donation, Van der Waal's forces, dipolar moment, and lipophilicity. For example, deuterated caffeine is known to elute faster in the lab on a gas chromatograph mass spectrometer. One might even imagine trying to capture nature's most elusive superbuzz by drinking it. Depending on which of caffeine's methyl groups were originally deuterated, the cytochrome 450 enzymes that kick off its transformation in your liver (ultimately to formaldehyde) would likely balk at the enzymatically more resistant C-D bonds. This will delay the formation of some metabolites, creating a relative preponderance of others.
To put this so-called 'deuterium switch' into the perspective of a larger business model, consider another devilish operation known in the pharmaceutical world as a 'chiral switch.' While often performed in much the same spirit as the deuterium shuffle, the creation of mirrorland molecules is arguably an even more significant, qualitative, and less predictable transformation. A recent radical report documents the creation of a 'reverse' DNA polymerase, presumably constructed from mirror image 'D' (or right-handed) amino acids. This polymerase has the ability to write mirror image DNA that winds to the left (as opposed to threading like a familiar right-handed screw).
The beauty of this emerging "looking-glass" world is that the southpaw polymerase has some unexpected talents—for one, it also writes RNA. Furthermore, researchers like George Church are already on their way to building mirror ribosomes that could be fed this mirror-RNA. Therapeutic mirror RNAs and proteins would have an unparalleled diplomatic immunity in the cell, rendering drugs made from them virtually untouchable by straight enzymes, in many respects upgrading the old Windows 32 cellular OS to 64-bits.
Recently, the other old hand in this new biochemistry, Craig Venter, asked Church during an interview if everything would still be copacetic—in other words, if mirror drugs and enzymes would really perform the exact same way in the mirror world. While drug companies may be salivating after Church's short-latency positive answer, there is some intriguing evidence that more subtle symmetry-breaking electron spin effects could be at play.
In one such conception, electrons originally in heterogenous spin states are released from an enzyme (like NADH synthase) and are subsequently filtered and polarized as they pass through chiral α-helix structures to the site of amino acid synthesis at the other end. This effectively produces "spin up" electrons that, if you can excuse the jargon, participate in the reductive reaction between α-oxo acid and ammonia with only L-amino acids forming according to the Pauli exclusion principle. In any event, to look in the mirror on the wall and see a biology that does not behave exactly like ours would seem to require some significant new parity breakdown in physics, to say the least.
Now, olfaction is probably the space where these deuterium switches and chiral switches most informatively converge to elucidate how receptors might operate. In fact, the authors explicitly highlight the fact that their histamine receptor model may have something to say about olfactory receptors. Importantly, both of these receptor classes belong to the so-called GPCR (G-protein coupled receptor) family that vertebrates use to detect odorants; half of our own 800 GPCRs are provisioned almost exclusively to olfaction.
The author's main comments, here, center on the aromatic groups of molecules, features that are typically associated with delocalized electrons. For example, the imidazole ring of histidine (histamine's the amino acid precursor) is aromatic at all pH values; four of its pi electrons form two double bonds and two from a nitrogen lone pair. The authors propose that a major fallout of deuteration is that the aromatic moiety shrinks the effective C–D distance relative to its C–H value. Aromatic C–H bonds act as proton donors and form weak hydrogen bonds with water molecules and proton acceptors at the receptor binding site.
In other words, that deuterated odorants would be a little different from nondeuterated odorants—something that has actually been appreciated for some time. These comments are pointed straight at recent experiments by Luca Turin, who has advanced the theory of molecular vibration sensing in olfaction in which the nose performs an analysis akin to your favorite benchtop device. Depending on the interpretation, that instrument might be part mass spectrometer, part IR spectrometer, and part scanning tunneling microscope. In particular, they question the conclusion of Luca's group that flies conditioned with progressively deuterated acetophenone could readily distinguished between the deuterated and nondeuterated varieties.
In response, Luca quickly noted a few problems. For one, he fairly observes, 'then how come the flies transfer learning from one deuterated compound to another, and from C-D stretch to C≡N ? By their lights, there should only be a difference in affinity. Why is there a commonality in smell character?'
Perhaps more pointedly, he notes that there are no aromatic CH groups in his deturerated musk experiments, only aliphatic groups—something the authors wisely avoid citing. Furthermore, the authors don't mention other work that shows very good correlations between vibrational spectra and agonist activity in histamine receptors.
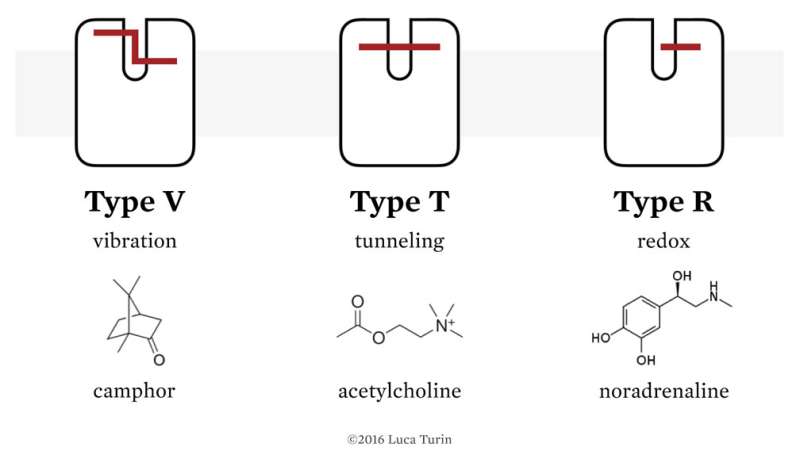
In a recent popular article, Luca has made a beginning toward a theory that puts the odor character back into the molecule. While not necessarily drugs, odors can be considered a special class of molecules with a much restricted receptor requirement. Due to inherent limitations in detecting volatiles, olfactory receptors can only expect to see molecules reflecting some trade-off in general stickiness and solubility—a compromise that makes specificity the frequent casualty. Luca proposed that GPCRs and their activators may be thought of as more like electronic components than the mechanical devices of the shape-based receptor paradigm. He suggests that cells could offer them in three styles—vibration (V), tunneling (T), and redox (R):
Type V receptors tunnel electrons across a gap that corresponds to an energy jump by binding a molecule that possesses one or more vibrations at the correct energy. Type T have the same circuit topology, but without an energy jump. The receptor is turned on when a molecule binds to it and includes a feature, such as a positive charge, that lowers the barrier to electron tunneling. Finally, type r receptors only have the output half of the circuit where the ligand brings in the electron, and then undergoes an oxidation step when bound.
Notably, GPCRs are frequently considered to be a predominantly Eukaryotic innovation. There is certainly evidence for GPCR precursors among the domains and motifs of proteins in lower life forms. However, bacteria generally go for more direct-acting receptors with efficient built-in ion channels as opposed to the laggy and protracted toggling of separate downstream ion channels actuated by messy G-protein cascades. For example, both bacteriorhodopsin and our rhodopsin belong to the 'seven transmembrane domain' family of proteins, but while rhodopsin is a GPCR, the ancient light-powered bacterial ion pump is probably not.
Why is this the case? If the primary job of sensory neurons is simply to encode incoming information into spikes, then what could be better than speedy ligand gated ion channels? One hint is the observation that if mitochondria generated or otherwise quickly fell out of the advent of eukaryotism, and GPCRs were an integral part of that transition, then the expected intracellular effect from GPCRs might be direct control of the locally resident mitochondria.
As possible counterpoint, here, one might point to those rare birds, the infinitesimal fairy flies that inexplicably jettison away much of their own neuronal nuclei and mitochondria and basically run on fumes till they expire. Such creatures might still sense and smell, but how well do they really do it?
More information: Mojca Kržan et al. The Quantum Nature of Drug-Receptor Interactions: Deuteration Changes Binding Affinities for Histamine Receptor Ligands, PLOS ONE (2016). DOI: 10.1371/journal.pone.0154002
Journal information: PLoS ONE
© 2016 Phys.org




















