May 23, 2016 report
Enzymes found that can tear down bacterial biofilm walls
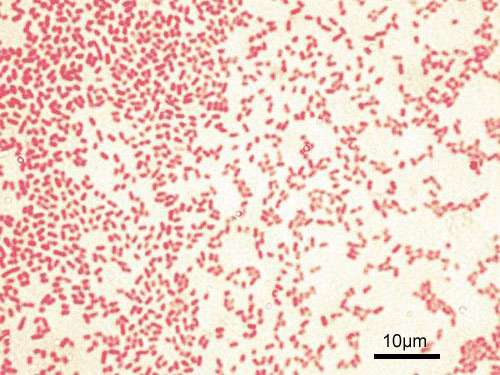
(Phys.org)—A team of researchers from the U.S. and Canada has identified two enzymes that have proven able to break down bacterial biofilms, allowing antibacterial agents to more effectively kill their targets. In their paper published in the journal Science Advances, the team describes their study of the structure of biofilms and how that led to the identification of the enzymes that are able to break them down.
As bacteria continue to develop resistance to drugs meant to kill them, one of the areas of research has been the biofilms that the bacteria build to protect themselves against attacks by the immune system. Some chemicals have been found that disrupt the biofilm building process, but what has really been needed is something that can tear the walls apart once they have been built, allowing antibacterial agents to gain easy access to the bacteria they are meant to kill. In this new effort, the researchers report that their study of the structure of such biofilms and the means by which they are built and maintained by bacteria, revealed a possible way to use enzymes that are a part of the process, to instead tear them apart.
In looking at the biofilms, the researchers discovered that two enzymes PslG and PelA, which are used by the bacteria as part of wall building, serve as a means of creating sugar polymers, which serve as building blocks of a sort—but they also help prevent the buildup of chains of sugars inside the cells of the bacteria. It occurred to the researchers that because the biofilm walls also have a chained sugar component, the same enzymes might cause wall degradation.
To test this idea, the researchers applied Pseudomonas aeruginosa bacteria to cultures in dishes in their lab, waited for them to build their biofilms, and then applied synthetic forms of the enzymes. The researchers report that the enzymes went to work eating the sugar in the biofilm walls, causing the walls to melt away, leaving the bacteria without their coating of protective goo.
The finding by the team is just the first step in looking into the possibility of using the enzymes to treat infections in humans, of course, a lot of work will need to be done to show that they do not cause harm, though there is cause for optimism—when they were applied to infected human lung cells, the researchers could find no damage due to the enzymes.
More information: P. Baker et al. Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms, Science Advances (2016). DOI: 10.1126/sciadv.1501632
Abstract
Bacterial biofilms present a significant medical challenge because they are recalcitrant to current therapeutic regimes. A key component of biofilm formation in the opportunistic human pathogen Pseudomonas aeruginosa is the biosynthesis of the exopolysaccharides Pel and Psl, which are involved in the formation and maintenance of the structural biofilm scaffold and protection against antimicrobials and host defenses. Given that the glycoside hydrolases PelAh and PslGh encoded in the pel and psl biosynthetic operons, respectively, are utilized for in vivo exopolysaccharide processing, we reasoned that these would provide specificity to target P. aeruginosa biofilms. Evaluating these enzymes as potential therapeutics, we demonstrate that these glycoside hydrolases selectively target and degrade the exopolysaccharide component of the biofilm matrix. PelAh and PslGh inhibit biofilm formation over a 24-hour period with a half maximal effective concentration (EC50) of 69.3 ± 1.2 and 4.1 ± 1.1 nM, respectively, and are capable of disrupting preexisting biofilms in 1 hour with EC50 of 35.7 ± 1.1 and 12.9 ± 1.1 nM, respectively. This treatment was effective against clinical and environmental P. aeruginosa isolates and reduced biofilm biomass by 58 to 94%. These noncytotoxic enzymes potentiated antibiotics because the addition of either enzyme to a sublethal concentration of colistin reduced viable bacterial counts by 2.5 orders of magnitude when used either prophylactically or on established 24-hour biofilms. In addition, PelAh was able to increase neutrophil killing by ~50%. This work illustrates the feasibility and benefits of using bacterial exopolysaccharide biosynthetic glycoside hydrolases to develop novel antibiofilm therapeutics.
Journal information: Science Advances
© 2016 Phys.org