The rise and fall of the antibiotic age
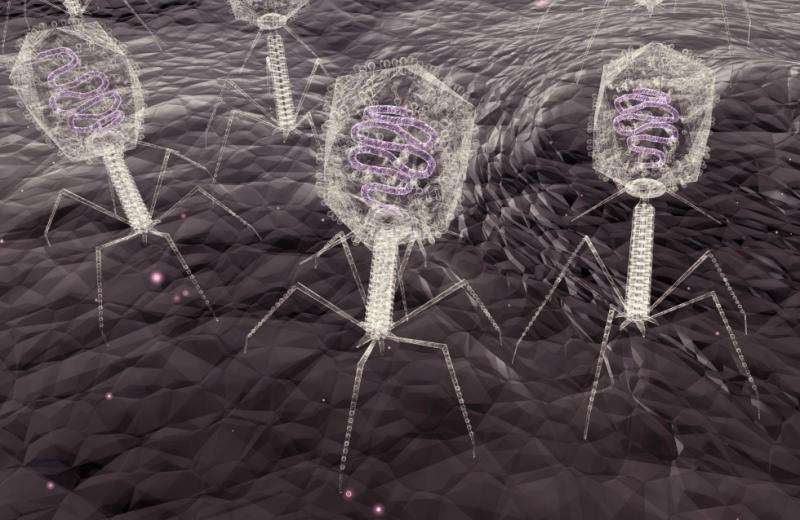
It's a dog-eat-dog world, but in the lab of University of Alberta bacteriologist Jon Dennis, it's actually virus-eat-bacteria.
Over the span of billions of years, viruses and bacteria have waged a cyclical war, co-evolving to one-up each other. Bacteria evolve to prevent viral infections; viruses, in turn, counter to find a way to infect and kill bacterial cells.
Dennis and U of A mechanical engineering colleagues Warren Finlay and Reinhard Vehring hope to harness this co-evolution and defeat some of the deadliest antibiotic-resistant bacteria known to exist. The failure of antibiotic drugs due to resistance is a very real and growing threat that, if left unchecked, could kill 10 million people by 2050, according to a recent analysis from the United Kingdom.
"Why not use all of the ingenuity that's gone into the evolution of viruses against bacteria and think about using those as a drug, instead of a chemical?" explains Dennis, a professor at the U of A's Department of Biological Sciences.
Two bacteria, Pseudomonas and Burkholderia, are of particular interest to Dennis. Both are deadly to people with cystic fibrosis, a genetic disease that can result in a buildup of mucus in the lungs, where bacterial pathogens take refuge.
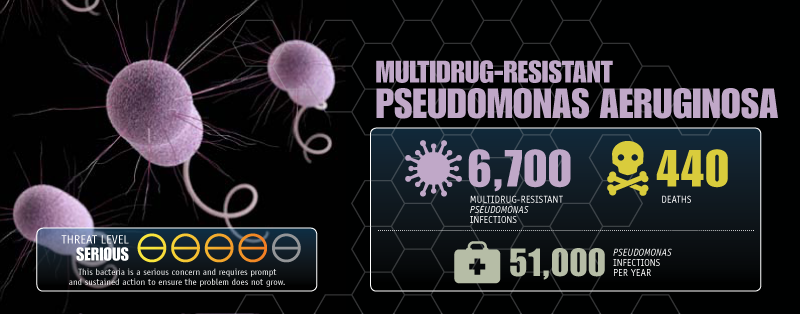
"These bacteria are highly antibacterial resistant. Once a patient gets an infection, you can never clear it—ever," explains Dennis. "The patient lives with this infection the rest of their lives."
In 2007, Dennis teamed up with Finlay, who runs the Aerosol Research Lab of Alberta in the Department of Mechanical Engineering and specializes in improving the delivery of drugs to the lungs. Finlay and ARLA routinely collaborate with researchers and companies around the world on developing inhaled pharmaceutical aerosols for diseases such as asthma.
One of their most recent collaborations involves bacteriophage therapy as a potential treatment for lung infections. Bacteriophage, which literally means "eater of bacteria," involves harnessing the evolutionary wizardry of viruses to destroy antibiotic-resistant bacteria.
The research team have introduced bacterial viruses, called phages—which are not alive but do possess genetic material that allow them to evolve—to a target bacterium. Once inside a bacterium's cell wall, phages use the bacterium's own energy sources to continuously replicate until the cell bursts open from the additional phage parts. Once that happens, this new generation of phages seek the next bacterium to invade and destroy until the infection is eventually cleared.
In effect, Dennis and Finlay are turning back the clock and taking a page from pre-antibiotic-era scientists to find solutions to antibiotic resistance.
French-Canadian scientist Felix d'Herelle co-discovered bacteriophages before the First World War and experimented with their use as a treatment for dysentery. (He even successfully treated a 12-year-old boy in 1919 after he volunteered himself as one of the test subjects to ensure it was "safe.")
d'Herelle would later manufacture several commercial phage therapeutics, as would others in the United States such as the Eli Lilly Company, which in the 1940s developed phage products targeting staphylococci and E. coli, among others, though the effectiveness was cast in doubt.
The former Soviet Union and Poland explored phage therapies into the 1990s, but the rise of antibacterial medications after the Second World War saw research into phage therapy largely ignored everywhere else.
Rising resistance and the post-antibiotic era
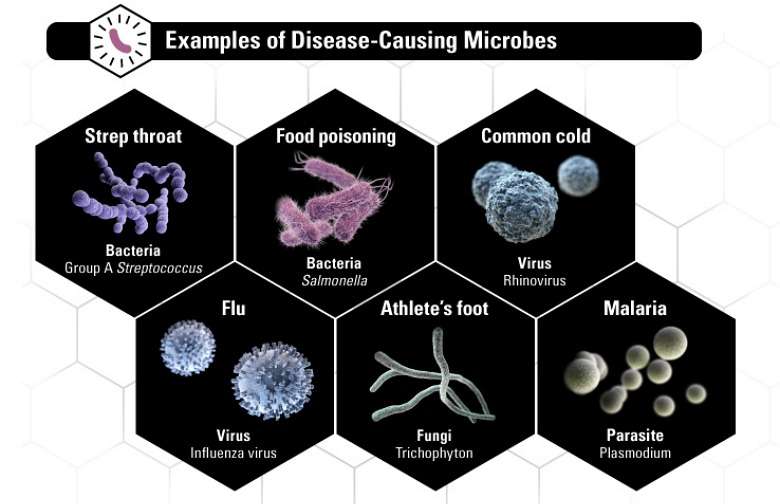
That's starting to change, particularly with the rise of antimicrobial resistance—a broader term that includes bacteria, fungi, viruses and parasites able to resist medicines. The World Health Organization calls it a serious and growing threat to global public health, which puts "the achievements of modern medicine at risk."
The threat has become so serious, some scientists argue we have already seen the end of the antibiotic era—where even a common infection can kill. The U of A's Lynora Saxinger says it's too soon to make such as declaration—at least for now.
"I don't think we are in quite in the post-antibiotic era, but I do think we are heading in that direction," says Saxinger, an associate professor in the Division of Infectious Diseases who has studied antimicrobial resistance, including as past chair of the Association of Medical Microbiology and Infectious Disease Canada's Antimicrobial Stewardship and Resistance Committee.
Saxinger co-wrote a 2014 report for the National Collaborating Centre for Infectious Diseases (NCCID) that examined antibiotic use in Canada and how well we are keeping tabs on antimicrobial resistance. What they found was poor co-ordination between provinces and, unlike the European Union, little in the way of national data—especially data that drills down to the hospital and community level. Part of the problem is that Canada is geographically vast and culturally diverse, and has multiple health systems.
Among its recommendations, the report calls for better surveillance to look for patterns in antibiotic use and resistance—information for action. Antimicrobial resistance directly correlates to antibiotic use; countries with higher rates of use or even self-medication such as Spain, Italy or the Balkan countries tend to have higher rates of resistance.
"If you don't know how much resistance you have, you can't really assess any kind of intervention to reduce resistance," Saxinger explains.
Heeding a 70-year-old warning
Antimicrobial resistance is not a new phenomenon; bacteria have adapted to their habitat to find ways to survive for billions of years. In a 2008 study, Russian researchers found that ancient bacteria strains—some millions of years old—uncovered in Siberian permafrost proved resistant to multiple drugs, including tetracycline, streptomycin and chloramphenicol—all antibiotics used by modern medicine.
Perhaps the earliest warning about resistance during the antibiotic age came in 1945, when Sir Alexander Fleming accepted the Nobel Prize for discovering penicillin. In his speech, Fleming warned that the "time may come" when penicillin is so readily available that it could be administered improperly, giving rise to resistance.
The intensive use of antibiotics during the last 70 years has both patients and physicians accustomed to turning to such treatments, whether or not they're the best option. In effect, we are helping bacteria become better at resisting.
"The uptake of antibiotics and love of antibiotics has been so strong that people who become ill want to get better as quickly as possible," says Saxinger. "Many don't realize the risks of adverse events, of how we are changing bacteria … the patient is so busy and wants to get better, all they want is the antibiotic. It's very hard for the physician to battle that."
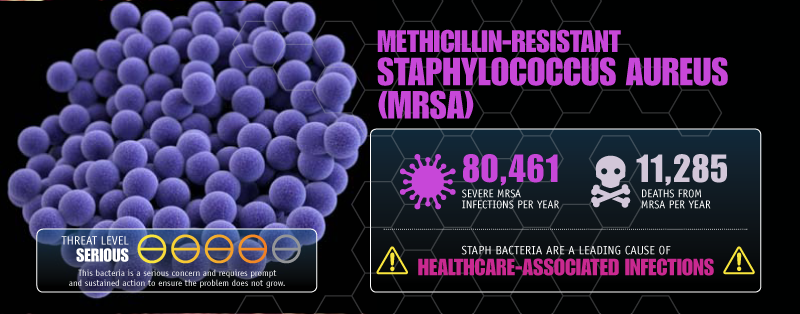
In Canada, antibiotic resistance has fundamentally changed patient outcomes, Saxinger notes. Staph infections from methicillin-resistant strains of Staphylococcus aureus, once a rare event in hospitals, now affect one-quarter of patients, she says. That has resulted in physicians turning first to more toxic antibiotics that are still effective against resistant strains.
"The antibiotics we use are often second or third line—more toxic than we like," she says. "It can be a really challenging problem."
Globally, the news is just as sobering. According to the WHO, there were about 480,000 new cases of multidrug-resistant tuberculosis in 2013, spreading to 100 countries.
Neisseria gonorrhoeae—the nasty bug responsible for gonorrhea, a sexually transmitted disease making a comeback—has become resistant to even "last resort" antibiotics such as cephalosporins. With no new drugs in development, there's a real risk gonorrhea could become untreatable.
Clostridium difficile, a so-called superbug that often spreads in hospitals and is potentially fatal, is on the U.S. Centers for Disease Control and Prevention's urgent threats list, responsible for 15,000 deaths in that country.
Klebsiella pneumoniae, which causes pneumonia in hospital settings, has spread to all parts of the world.
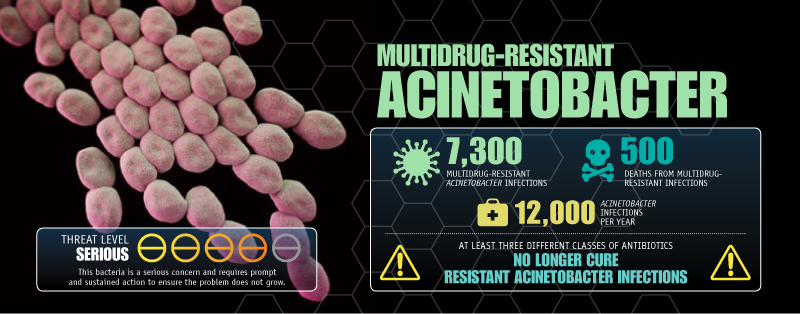
Another bacterium on the CDC's threat list, Acinetobacter baumannii, has become resistant to multiple drugs. In 2012, infection from Acinetobacter led to a death at Edmonton's Royal Alexandra Hospital.
Where are the new antibiotics?
When penicillin was mass-produced for the first time by the Allies during the Second World War, its impact was immediate. The wartime death rate from bacterial pneumonia fell to less than one per cent; a generation earlier, 18 per cent of First World War soldiers died.
In the subsequent decades, new antibiotics were developed that saved millions of lives. The "war" was thought to be won. But, just as they have for three billion years, bacteria continued to adapt to their environment, developing genes that make resistance possible to even multiple drug combinations.
This rise of resistance has been accompanied by stagnation in the development of new antibacterial drugs, something Dennis blames on the economics of drug manufacture. Since the 1960s, only two new classes of antibiotics have been developed and even those are starting to fail due to the rise of resistance.
"Once you cure an infection using antibiotics, that drug is no longer required, as opposed to lifestyle drugs that patients continually need to take. So, a lot of industry has moved away from antibiotic research," explains Dennis.
Back to the future
In late 2014, Dennis and Finlay were awarded a U.S. patent for their phage composition and delivery system.
Dennis identified phages to target Pseudomonas and Burkholderia bacteria but lacked a mechanism for ensuring their delivery in the lungs. He supplied the phages in liquid form while Finlay provided the engineering know-how to essentially create a phage inhaler. Aerosolizing the liquid requires using fairly destructive methods, so care was needed to avoid destroying the phage samples, Finlay explains.
"We proceeded with several different approaches to aerosolization. Some worked better than others," he adds. Eventually, they landed on powder inhalers, like the kind used to treat asthma. "To take this aqueous formulation and turn it into a powder—phages don't like being turned into a powder; trying to figure out how to protect them was a challenge."

Their colleague Vehring came to the U of A after decades in pharmaceutical engineering where he worked at several San Francisco-area companies developing aerosolized therapies such as the world's first inhalable insulin, Exubera, and the needle-free flu vaccine FluMist. As an engineer who designs microparticles that can be dispersed through aerosolization, Vehring says he admires phages.
"As an engineer, I see these things as highly sophisticated nanomachines in a way," he says.
Vehring says he turned to academia to work on challenges that fall outside the profit-driven interests of the pharmaceutical industry, and sees potential in phage therapy in treating respiratory infections such as multidrug-resistant tuberculosis, which killed 1.5 million people worldwide in 2014.
"If academia doesn't do that work, who is going to do it? There is no other function of society that would take that. Even non-profit organizations cannot do that," Vehring says.
Though phage therapy offers great potential to help solve the antibiotic crisis, viable treatments are still years away. As is the case with developing chemical drugs, phage treatments need to go through multi-phase clinical trials—extensive and expensive testing that regulatory agencies such as Health Canada require to prove a drug is safe.
With costs on the order of hundreds of millions, Dennis says it's impossible for the U of A team to advance their phage research much further without sizable third-party investment. Though countries like the U.K. and Australia are funding this kind of basic research, that isn't the case in Canada.
"Canada has been very reluctant to fund this kind of research," Dennis says. "Until we get a drug company interested or a national funding agency puts out a call for either team grants or something related to basic research in phages, then really we're just spinning our wheels."
It's particularly frustrating for Dennis, who says he's regularly contacted by families affected by cystic fibrosis—and even some hospitals—inquiring about their technology.
"I'd dearly love to give them hope and medicine, and perhaps cure them or save them," he says, but adds it's clearly impossible without funding and regulatory approval. "I've seen many people die that, potentially, we could have helped. I'm not going to say we could have saved them outright, but perhaps phage therapy could have helped."
Engaging government key to winning the war
Though much progress has been made in understanding the scope of antimicrobial resistance, winning the war will require much greater vigilance and a united front among provincial health authorities and between countries.
Surveillance is at a "critical point," warned Saxinger and her colleagues in their NCCID report. More effort is needed to improve co-ordination not just between governments and health authorities, but also with veterinarians and food animal producers, which also use antibiotics and have even less sophisticated methods of surveillance, she says.
In addition to improving surveillance, more work is needed to educate the public and physicians about antimicrobial resistance. Public education campaigns such as "Do Bugs Need Drugs?" have been shown to help, Saxinger notes. Developed in Alberta and introduced in British Columbia, the campaign aims to reduce the spread of infectious diseases through handwashing and the reduction of unnecessary prescribing.
Average Canadians can help by understanding every action they take has a direct impact on their environment.
"Everyone has an antibiotic footprint just like you have a carbon footprint. Everything you do contributes to ecology of it," Saxinger explains. "Segments of the public understand that quite well, it's just not consistent. There are still some diehard antibiotic users and prescribers."
And just as government has a role in changing behaviour, more can be done to ensure research discoveries actually translate from the bench to the bedside. Given the scope of what's at stake, Vehring says, government should step up by offering incentives to the pharmaceutical industry to take interest in developing less profitable drugs, or re-examine the regulatory approval process to bring drugs to market faster and reduce costs.
"If there's a global health emergency, you can't just say develop me a new drug in the next half-year. It's impossible. You have to be more proactive, and that is where I see the risk," he says. "We do have an issue here and it should be addressed."
More information: A. Sulakvelidze et al. Bacteriophage Therapy, Antimicrobial Agents and Chemotherapy (2001). DOI: 10.1128/AAC.45.3.649-659.2001
Journal information: Antimicrobial Agents and Chemotherapy
Provided by University of Alberta