Asynchronous cell cycle phase key to critical stage of animal embryonic development
Cell division of a mother cell to produce two identical daughter cells is known as mitosis. This underlies the early embryonic development of sea squirts, when it changes from being highly synchronized to displaying a distinct spatial pattern that accompanies neural tube formation. However, researchers did not understand what controlled this switch in mitotic timing. A recent study from the University of Tsukuba reveals that the change represents the beginning of a mitotic wave that passes from the back to the front of the embryo following loss of asynchrony of a gap phase of the cell cycle, which had until now masked an asynchronous synthesis phase. The study was reported in Developmental Cell.
Animal embryos undergoing early development rapidly increase their cell number through quick, successive rounds of cell division that involve DNA synthesis and mitosis without the gap phases usually seen in eukaryotic cell division. Gap phases occur within the cell cycles once a particular number of divisions have occurred. At this stage, the transitions between Gap 1 (G1) and Synthesis (S), and Gap 2 (G2) and Mitosis (M) are controlled by cell cycle proteins such as cyclins and the enzyme Cdc25.
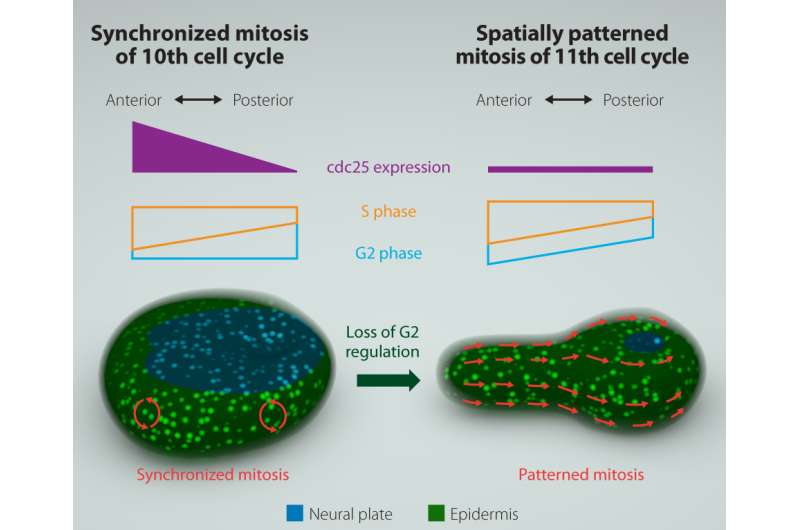
The simple nervous system of sea squirts (ascidians), which are marine chordates, is often used to model the embryonic development of complex vertebrates. In ascidians, the embryo's outer layer (the ectoderm) forms a neural tube that joins up in a zipper-like process from back to front. This process corresponds to the 11th cell cycle of epidermal cells, which has an extended G2 phase regulated by reduced expression of the gene encoding Cdc25.
To understand how the spatial pattern of mitosis is achieved, the researchers fluorescently labeled a DNA replication protein to analyze mitotic timing through time-lapse imaging. Focusing on differences between the 10th and 11th cell cycles, they observed a temporary G2-phase compensatory mechanism. "In the 11th cell cycle, the S-phase length increases in a posterior-to-anterior direction, which is responsible for the observed spatial pattern of 11th mitosis," corresponding author Yosuke Ogura explains. "The S-phase length difference is identical in the 10th cell cycle, but here the length of the G2 phase progressively increases in the front-to-back direction, compensating for and masking the asynchrony of the S phase. The compensation (or masking) is necessary for development, because the embryos fail to form proper neural tube when the compensation was artificially removed."
Through a range of experiments, the researchers also demonstrated that synchrony of the 10th mitosis requires asymmetric expression of the cdc25 gene from the front to the back of the embryo, which is itself controlled by GATA and AP-2 proteins that regulate gene expression. "The loss of GATA and AP-2 expression downregulates cdc25 gene transcription," coauthor Yasunori Sasakura says. "This removes the compensatory relationship between the lengths of S and G2 phases." Without this counteracting mechanism, the 11th mitotic division occurs in a posterior-to-anterior wave that coordinates with morphogenesis.
More information: "Developmental Control of Cell-Cycle Compensation Provides a Switch for Patterned Mitosis at the Onset of Chordate Neurulation" Developmental Cell, DOI: dx.doi.org/10.1016/j.devcel.2016.03.013
Journal information: Developmental Cell
Provided by University of Tsukuba