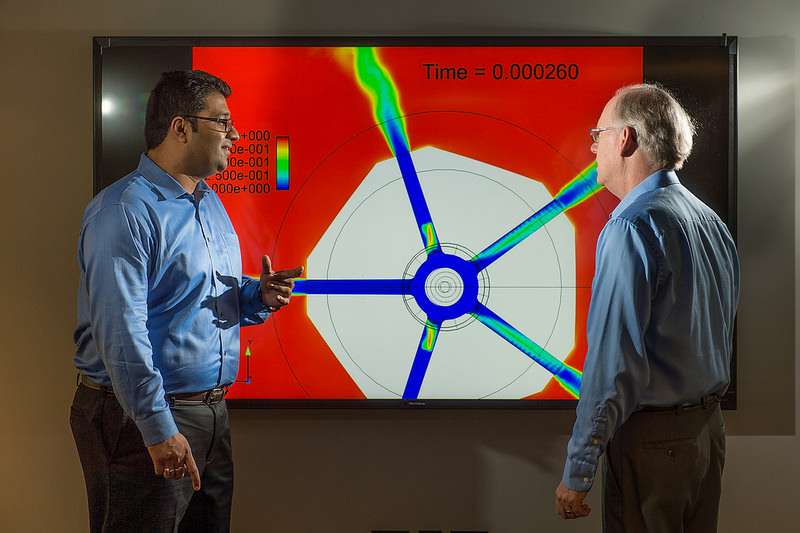
Sibendu Som (left) and computational scientist Raymond Bair discuss combustion engine simulations conducted on Argonne’s Mira supercomputer, with the aim of gaining further insight into the inner workings of combustion engines.
A billionth of a second: That's how quickly some of the most critical chemical reactions of combustion occur.
Argonne chemist Stephen Pratt leads the Gas-Phase Chemical Dynamics Group at Argonne. His team, which includes a dozen Ph.D. scientists, several postdocs, and numerous visiting students and researchers, is trying to gather as much information as they can about something that lasts only an instant.
Their focus: understanding the chemistry of combustion.
Combustion chemistry in the cylinder of an engine takes place in the gas phase. Individual reactions can be considered at the molecular level.
"If you think about combustion in an engine with fuel, it seems like a simple process but it is actually very complex," Pratt said.
This is true of even the simplest process, one in which hydrogen and oxygen combust, he said.
The reaction of oxygen (O2) with two hydrogen (H2) molecules results in the production of two water molecules (H2O). But when they combust in real life, dozens of other things occur.
For an accurate description of this process, 25-30 different reactions must be considered, even though only two chemical elements are involved.
Complexity, by the gallon
Current fuels, such as those that power our cars, provide an even greater challenge.
The gasoline we use each day on our way to work is actually a mixture of more than 1,000 different chemicals. When it burns, the number of chemical species and reactions multiply dramatically.
"If you try to model something like that, you have to consider the rates of all the reactions and how those rates depend on temperature and pressure, among other factors," Pratt said. "A tremendous amount of information needs to be built into these models to make them sufficiently accurate for quantitative predictions.
"Some of the important reactions involve very reactive fragments of these fuel molecules that live for a very short time," Pratt said. "They are incredibly difficult to study experimentally."
If accurate reaction rates and energetics could be determined by theoretical calculations—rather than through experimentation—it could provide a solution to this problem.
Thom Dunning, now at the University of Washington, initiated Argonne's combustion chemistry effort in the late 1970s. His vision was that theoretical chemistry would one day be good enough to calculate all of the necessary information, allowing the construction of predictive combustion chemistry models from first principles.
"Forty years ago, that seemed far-fetched," Pratt said. "Today, we are closer than ever to making it happen."
Since the beginning of this effort, Pratt said, Argonne has pulled together a balance of experimentalists and theoreticians to study reaction dynamics and rates.
"The constant interplay between these researchers has been invaluable for understanding the chemistry and improving the theoretical methods," Pratt said.
Their research is broad in scope. While some focus on chemical energetics, others study the dynamics and rates of the reactions, as well as the rates of related processes like energy transfer between colliding hot molecules.
Once the individual reactions are characterized, they are assembled into larger chemical models for selected fuels.
Techniques are also being developed to improve the predictivity of these models through improvements of selected rate data.
Predictive science
After decades of research, theory alone can now reproduce experimental results for many classes of reactions, and can also make accurate predictions for some reactions that are not easily amenable to experimentation.
While considerable challenges remain, Pratt said, the goal of predictive chemistry models is almost within reach.
"We are beginning to see a light at the end of the tunnel," Pratt said. "It's really exciting."
Ultimately, this capability will not only aid the development of improved engines and fuels, but also speed the introduction of alternative renewable fuels into the commercial market.
Argonne's efforts in this area are widely recognized. Three of its scientists—Argonne Distinguished Fellows Lawrence B. Harding and Albert F. Wagner and senior chemist Joe V. Michael—were recently feted by the Journal of Physical Chemistry A with a special issue in honor of their 100 years of combined work in combustion kinetics.
"The three of them are terrific scientists," said Pratt. "This is one of the top journals in our field, and this special issue highlighted the importance of Larry, Al, and Joe's contributions to combustion chemistry."
Simulations
Sibendu Som, a mechanical engineer at Argonne, develops predictive tools to simulate the processes taking place inside internal combustion engines.
"Engines are very forgiving," said Som, who joined the laboratory in 2009. "Burning is not the challenge. You can put in any fuel and it can burn. It's the efficiency of combustion that is the challenge. How do we get better mileage out of our vehicles? And how do we do it cleaner than it's been done before?"
As the principal investigator on the simulation program, Som uses computing clusters and the lab's supercomputer to test theories about combustion that would make the process far more efficient.
"In the past, engine simulations used only very simple models, which were not predictive," he said. "What we are trying to do is use complex models that capture more of the physics in terms of fuel spray and combustion. By using the clusters and the supercomputer, we can run tests and simulations with a 24-48 hour turnaround, which would be impossible otherwise. And this technology allows us to reduce the uncertainties in the simulation so the outcome is far more precise. We can do things now we could not do five years back."
As part of that effort, Argonne has entered a cooperative research and development agreement with a leading company that designs, manufactures, distributes, and services diesel and natural gas engines as well as another company that is a leader in computational fluid dynamics software.
Som's work allows him to test for ignition delay, heat release rate, and emissions, among other essential components of combustion.
"My team is responsible to help and optimize gasoline combustion," he said. "In order to do that, we have to answer several questions, like 'When should the fuels be injected? And at what angle?' If it is not injected at the correct angle, it will not burn properly."
Molecule by molecule
Douglas Longman, a section manager in Engine Combustion Research at Argonne, has been with the laboratory for 17 years. His team is responsible for a broad range of experimental and computer simulation work, including fundamental combustion research—where scientists use a rapid compression machine to mimic the same type of conditions found in an engine, but in a far more controlled fashion—to the study of the basic chemistry behind each explosion.
The mystery, Longman said, lies at least in part with the gasoline itself. The octane rating of 87 or 91 that we see at the service station doesn't tell scientists enough about how the fuel will perform, so they rely on something called a rapid compression machine to fill in the blanks.
"Gasoline is composed of hundreds of different components, and each has its own unique combustion properties," Longman said. "The rapid compression machine shows us the interaction of different types of molecules. The real fuels are then compared to our simplified mixtures to understand their characteristics."
Beyond that, scientists at Argonne also study exhaust emissions and the performance and ignition of different engine configurations.
"We can do imaging inside the combustion chamber, which helps us get a better experimental data set that we can compare to the simulations that other scientists, like Sibendu Som, do on the computers," Longman said. "The imaging allows us to account for temperature, the distribution of fuel and air mixture, whether it is well mixed or has pockets of too much or too little fuel."
Diesel engine, gasoline fuel
One of the key projects in this area is a new engine combustion concept: gasoline compression ignition.
"It's kind of a combination of a diesel engine and a gasoline spark ignited engine, which is what most U.S. cars have," Longman said. "Those are two different combustion approaches. They each have their own benefits."
A diesel engine combustion system is very fuel-efficient but creates too much pollution, generating nitrogen oxides and soot. Gasoline engines—which are spark-ignited—are cleaner-burning, but are not as efficient. In terms of miles per gallon, you burn more fuel with gasoline than with diesel, Longman said.
"Basically, the gasoline compression ignition is trying to use the gasoline fuel in a diesel-like combustion process," he said. "We are putting gasoline in a diesel engine and are able to operate it by controlling how the fuel is introduced into the combustion chamber."
And by doing that, scientists are hoping to gain the high efficiency of the diesel process and the low emissions of the gasoline fuel.
"We have been working in this area for four to five years," Longman said. "And we've made a lot of progress. We imagine this could be available to consumers in about 15 years."
Stationary natural gas engines
The lab also is studying stationary natural gas engines, which are the same type of engine found in our cars, though much larger and connected to a generator. They would supply electricity to the power grids.
"We have been working for years on how to make those engines more efficient," Longman said. "There are a couple of ways to do it, but both tend to make it more difficult to ignite the natural gas and air mixture. The spark plugs don't work well under some of these conditions. We are using laser igniters to ignite the fuel and air mixture instead of a spark plug."
The lab also is researching locomotive-sized diesel engines.
"As with almost all of our programs, the focus is on better fuel efficiency at lower emissions levels," he said.
Argonne computational scientist Ray Bair has twice worked for the laboratory. During his first tour, he was in the theoretical chemistry group and focused on combustion research. He currently works in the computing, environment and life sciences directorate, where he serves as the chief computational scientist for applications.
"My background is in computational molecular science, but what I do now draws on more than a decade of interactions with computational science and engineering teams," he said. "I work with research teams across the lab to help develop computing strategies and build partnerships to try and solve some of the key computational challenges in energy research."
With a focus on high-performance computing, he manages the lab's internal supercomputer center, where he works with dozens of scientists
in varying fields of research.
He is currently part of a multidisciplinary team helping with the plans for the Virtual Engine Research Institute and Fuels Initiative, or VERIFI, which brings together expertise from all four directorates at Argonne.
"If you look at the common practices of the engine industry in terms of the accuracy of their models and how predictive they are, there is an opportunity to do a lot better with supercomputers," he said. "VERIFI is just one example of how an institution like Argonne can work together with industry to solve these problems."
What is to come
Historically, consumers had a narrow selection of fuels, e.g., gas or diesel. But looking forward, the worldwide fuel mix is becoming ever more varied. As biofuels are introduced—they come from different sources and burn differently—engines will need to accommodate a wider range of fuel properties.
Having accurate models of engine operations gives us a way to accommodate this diversity in the fuel mix—and improve the safety, efficiency, and cleanliness of the combustion engine.
Journal information: Journal of Physical Chemistry A
Provided by Argonne National Laboratory