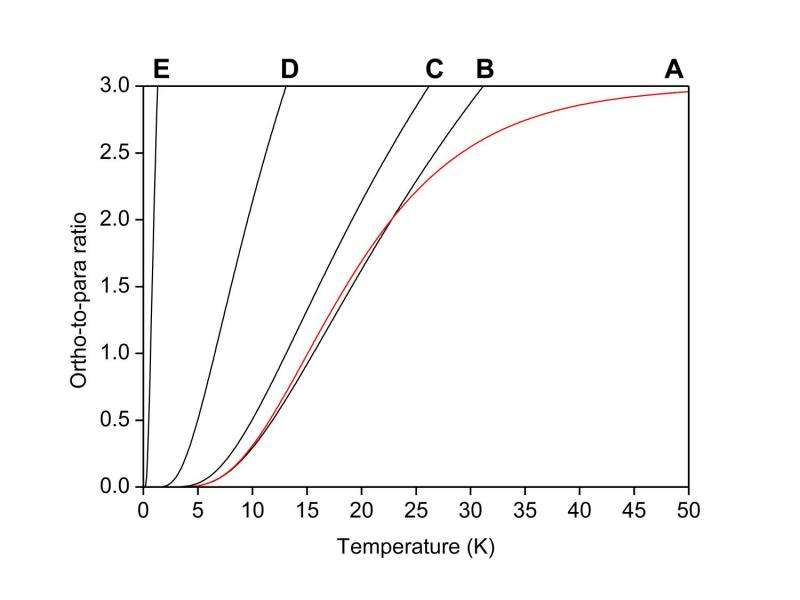
OPR of H2O as function of temperature. Curve A is calculated from Eq. 1 with the gas-phase constants. Approximated curves B to E are calculated from Eq. 9 with DE values of (B) 23.8 cm−1 (gas-phase), (C) 20 cm−1 (H2O in Ar matrix), (D) 10 cm−1, and (E) 1.0 cm−1. Approximated curves B to E tend toward 9, not the statistical OPR value of 3, because only the lowest ortho- and para-rotational states are considered in Eq. 9, and thus the contribution of the rotational degeneracy is not cancelled as in curve A from Eq. 1. Details of Eqs. 1 and 9 in paper. Credit: Prof. Tetsuya Hama, Hokkaido University.
For the past 30 years, the significance of the anomalously low ortho-to-para ratios (OPRs) of gaseous water (H2O) in interstellar space has remained unknown. (In ortho hydrogen molecules, both nuclei spin in the same direction, while in para hydrogen the nuclei spin opposite directions.) Recently, however, scientists at Hokkaido University, Sapporo, Japan found that water desorbed (that is, released from or through a surface) from ice at 10 kelvin shows a statistical high temperature OPR of 3 rather than the lower values typically found, even when the ice is produced in situ by a known formation process of interstellar water known as O2 hydrogenation. This invalidates the assumed relation between OPR and temperature and requires a reinterpretation of the low OPRs will help elucidate the chemical history of interstellar water from molecular clouds and processes in the early solar system, including comet formation.
Dr. Tetsuya Hama and Phys.org discussed the paper he and his colleagues published in Science, starting with the challenges of showing that water desorbed from ice at 10 kelvin shows a statistical high-temperature ortho-to-para ratio (OPR) of 3 even when the ice is produced in situ by hydrogenation of O2, a known formation process of interstellar water. "Detecting ortho-H2O and para-H2O separately is the main difficulty in studying the H2O ortho-to-para ratio," Hama tells Phys.org. "Ordinary analytic techniques, such as electron or single-photon ionization, cannot distinguish them. We also need high sensitivity for detecting ortho- and para-H2O, since only a small amount of H2O is desorbed from ice following photoirradiation or thermal heating." In addition, Hama adds, it's necessary to produce a sufficient amount (10-20 monolayers) of water ice in situ on a substrate at 10 K. In their experimental setup, the researchers produce water ice by O2 hydrogenation (a chemical reaction between molecular hydrogen and another compound or element) at 10 K – but it requires seven hours. "These technical problems – that is, ortho- or para-state selective detection and in situ ice preparation – are the main reasons that studying the OPR of H2O desorbed from ice is challenging, and thus, the conventional proposal that the OPR is related to the condensation or formation temperature of ice has not been tested experimentally."
An interesting aspect of the study was the goal of clarifying the origin of the anomalous OPRs observed for interstellar H2O due to the role of gas-phase processes being poorly understood. "Our results suggest that the OPR of H2O desorbed from ice is the statistical value of 3 regardless of the formation process of the ice, so the anomalously low OPR of interstellar H2O may be an indicator of the gas-phase processes that the H2O experienced after desorption," Hama explains. "However, ortho-para conversion would not occur in the gas phase by radiation or nonreactive collisions, because they are spin-forbidden." Hama therefore believes that the gas phase ortho-para conversion of H2O may occur by chemical (proton-exchange) reactions, likely candidates of which are proton-exchange reactions with ions such as H+ [ortho-H2O + H+ → para-H2O + H+] or H3O+ [ortho-H2O + H3O+ → H3O+ +para-H2O]. These two reactions are almost thermo-neutral, Hama points out, but the reverse processes can be slightly endothermic and thus very slow – especially at low temperature – because of the energy difference of ortho and para H2O in the gas phase [∆E = 23.8 cm−1 (34.2 K)]. Thus, these reactions might lead to para-enrichment of H2O in cold (< 30 K) molecular clouds.
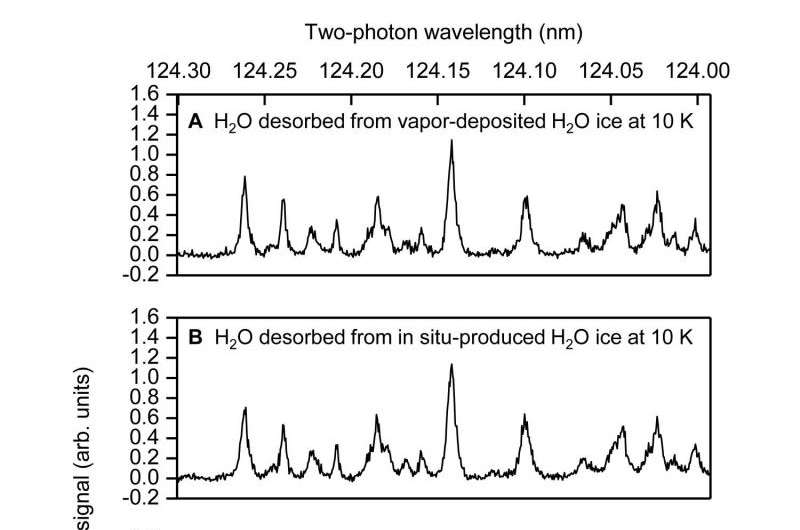
REMPI spectra of photodesorbed H2O after 157 nm photoirradiation of water ice at 10 K. (A) Vapor-deposited H2O ice. (B) H2O ice produced in situ by means of hydrogenation of O2. (C and D) Simulated spectra with Trot = 200 K and (C) Tspin = 200 K and (D) Tspin = 10 K. Indications (JKa,Kc) are rotational assignments, where “o” and “p” denote ortho and para, respectively. Trot and Tspin represents rotational and spin temperatures, respectively. Credit: Prof. Tetsuya Hama, Hokkaido University.
"Unfortunately, accurate rate coefficients for these reactions at low temperature are unknown, so quantitative estimation of the importance of these reactions is not possible for now. To study these gas-phase reactions, we have to treat ortho and para H2O separately (nuclear spin-state–dependent), but it is a challenge both theoretically and experimentally in current physics and chemistry."
Since ordinary analytic techniques cannot distinguish ortho and para H2O, the scientists addressed these challenges by employing the Resonance Enhanced Multi-Photon Ionization (REMPI) technique. Originally developed for spectroscopic investigations in the field of physical chemistry, REMPI allows ortho-para state selective detection of H2O by tuning the photoionization laser wavelength – and it has very high sensitivity. The researchers think that REMPI perfectly suits the study the OPR of H2O desorbed from ice, and have designed and built a novel experimental apparatus that enables both in situ ice production and REMPI detection of ortho- and para-H2O desorbed from ice at 10 K.
"Scientifically," Hama points out, "we suggest that the ortho-para nuclear spin physics of H2O in ice is totally different from that in the gas phase. The conventional proposal that the OPR is related to the ice formation temperature implicitly assumes that para-H2O is more stable than ortho-H2O in ice, as it is in the gas phase." However, Hama notes that the thermodynamic stability of para-H2O in the gas phase stems from the rotational energy difference between para- and ortho-H2O [(JKa,Kc=000 and JKa,Kc=101, respectively). In ice, both ortho- and para-H2O do not rotate by hydrogen bonds - meaning that unlike the gas-phase, the thermodynamic stability of ortho- and para-H2O should be comparable in ice at 10 K. Moreover, he adds, fast continuous ortho–para interconversion would occur in ice owing to intermolecular proton-magnetic interaction, which does not occur in the gas phase.
"I therefore think that the conventional proposal should reconsider these differences in ortho-para nuclear spin physics of H2O between the gas and ice phases. Our findings can also apply to other hydrogen-bonding systems such as NH3 in ice, so we can predict that the OPR of NH3 desorbed from ice would also be statistical, and cannot be used to deduce the surface temperature of interstellar dust."
One of the finding's key results was invalidating the assumed relation between OPR and temperature, thereby elucidating the chemical history of interstellar water from molecular clouds and processes in the early solar system, including comet formation. Moreover, regarding the OPR of H2O in a comet, the study explained why nearly all of the observed OPRs lie within 1 or 2 of the statistical value. "For the OPRs of H2O in cometary comae, the story is hopefully somewhat simpler." (A coma is the nebulous envelope around a comets' nucleus.) "The OPR of H2O should be statistical (=3) at desorption from the comet nucleus, not indicating the past formation temperature of the ice nucleus. In comae, the collision rate and the total number of collisions of H2O with other H2O molecules, ions, electrons, and dust grains are predicted to be too small to induce efficient conversion." Therefore, the observed OPRs of H2O in cometary comae would not greatly change from the statistical value of 3 at desorption from the nucleus, which explains why OPR values of H2O in cometary comae lie within 1 or 2 of the statistical value.
The paper also challenged approaches that assume that the OPR of water desorbed from ice is related to the ice formation temperature on the dust, and provided a reinterpretation of OPR's importance as a physicochemical tracer by considering gas-phase processes may clarify new or missed roles of interstellar H2O in star and planet formation. "The OPR has been a key observable in astronomy and planetary science," Hama tells Phys.org. "For example, the OPR of H2O in a comet has been considered a cosmogonic indicator" – that is, a finding based on the astrophysical study of the origin and evolution of the universe – "that gives the past formation temperature (30 K) of the ice nucleus in the solar nebula some 4.6 billion years ago. However, we experimentally disproved the conventional assumption of OPR's relation to temperature, such that it cannot be used to deduce the surface temperature of interstellar dust at H2O formation or condensation." In short, the researchers have made it necessary to develop a new interpretation of the anomalously low OPR of H2O.
"The origin of the anomalous OPRs is still an open question for now, but we can find a new direction in which the gas-phase processes may be important," Hama continues. "The OPR may be an indicator of the gas-phase nuclear-spin conversion processes that the H2O experienced, such as reactions with H+ and H3O+." This, in turn, may yield detailed information about the physical and chemical conditions – including temperature, the types of ions, and their abundances – of local environments where the gas-phase nuclear-spin conversion of H2O occurs. "Our finding is the first step towards understanding the significance of the OPR of interstellar H2O, which has been unknown for almost 30 years since its first observation. Approaches from quantum physics and chemistry as well as astronomy and planetary science are certainly needed for future studies, which is truly an interdisciplinary science."
Regarding next steps, Hama tells Phys.org that while in the present study, the researchers measured the OPR of gaseous H2O desorbed from ice by photoirradiation and heating, they could not measure the OPR of nascent H2O formed on ice, the nuclear spin conversion time of H2O on ice, and the nuclear spin state of H2O in ice. "These are still unsolved, challenging problems, but the nuclear spin conversion time could be measured with ortho- or para-H2O separation techniques." In addition, he continues, recent progress in observational studies in astronomy and planetary science has succeeded in providing detailed physical and chemical information – species, abundance and its spatial distribution, energy state (electronic, vibrational, rotational, nuclear-spin), isotope fractionations, and other metrics – about interstellar molecules.
"These can help us to understand the evolution of stars and planetary systems," he says. "However, to obtain a proper meaning from observations, we must understand the physics and chemistry of molecules in extraterrestrial conditions well. These are often extreme environments, such as very low temperature (10 K), and there are still many unknowns and unexpected phenomena. We would like to tackle the physics and chemistry in these extreme environments."
As esoteric as the team's research may appear, their findings are relevant to a host of other research disciplines. "Our results advance fundamental understanding of nuclear-spin conversion and the separation of nuclear spin isomers of molecules, both of which are important challenges in physics and chemistry. For example," he illustrates, "para enrichment of H2O has been studied for its application to highly-sensitive nuclear magnetic resonance (NMR) spectroscopy. However, our results imply that if H2O could be enriched in ortho or para states, it would become a statistical mixture (OPR=3) once it is incorporated into ice. This means that the isolation of ortho- or para-water in ice and its application to NMR study is quite difficult."
More information: T. Hama et al, Statistical ortho-to-para ratio of water desorbed from ice at 10 kelvin, Science (2015). DOI: 10.1126/science.aad4026
Journal information: Science
© 2016 Phys.org