They are wedge-shaped and can independently assemble themselves into supramolecular structures -- complex organized groups of multiple molecules. Credit: authors of the study
An international group of scientists from Russia, France, and Germany have developed ion-exchange synthetic membranes based on amphiphilic compounds that are able to convert the energy of chemical reactions into electrical current. The new development, described in the journal Physical Chemistry, Chemical Physics could potentially be used in fuel cells, and in separation and purification processes. The study was conducted by MIPT's Laboratory of Functional Organic and Hybrid Materials, which was opened in 2014.
Fuel cells consist of separate galvanic cells and their closest relatives are batteries (primary cells) and accumulators (secondary cells). Batteries convert the energy of the reaction between an oxidizing agent and a reducing agent, and stop working when these agents are used up. An accumulator stores electrical energy applied to it from an external source, converts it to chemical energy, and releases it again, thus reversing the process. A fuel cell, on the other hand, which is also an electrochemical generator, gets the materials that it needs to function from an external source. These materials are a reducing agent (usually hydrogen, methanol or methane) and an oxidizing agent, oxygen. Providing these materials from an external source means that electricity can be obtained from a fuel cell continuously without having to stop for recharging as long as the parts of the cell are in working order.
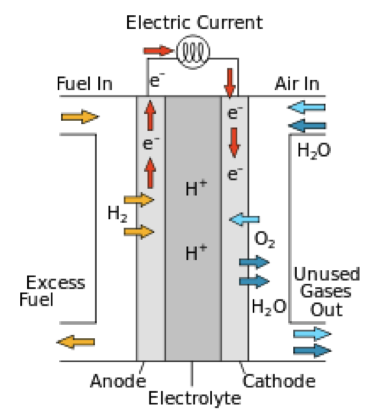
The main elements of this generator are a cathode and an anode, separated by an ion-exchange membrane.
At the cathode, the reducing agent is dissociated—an electron is separated from a hydrogen molecule (or another fuel) and thus, a positively charged hydrogen ion, a proton, is formed. The membrane allows protons to pass through, but retains the electrons—these particles are forced to take the "long route" through an external circuit. Only once they have passed through this circuit (the device that the fuel cell is powering) can they reach the anode, where they find oxygen and the protons that passed through the membrane to combine and form water. The electrons, which are forced to go around the membrane, create a current in the external circuit that can be utilized.
Why do we need fuel cells and why are they not used more widely?
Fuel cells use the same fuel that can be burned in conventional internal combustion engines producing the same basic products—water vapour in the case of hydrogen, and water vapour with carbon dioxide in the case of organic fuel. However, compared to a traditional engine, a fuel cell has at least two advantages: First, the process takes place at a lower temperature without a number of harmful emissions such as nitrogen oxides; secondly, fuel cells can have a much higher level of efficiency. Petrol and diesel generators are limited by thermodynamic laws (they do not allow an efficiency coefficient of more than 80 percent, for example), but such laws do not apply to fuel cells.
They are wedge-shaped and can independently assemble themselves into supramolecular structures -- complex organized groups of multiple molecules. Credit: authors of the study
In a number of technological applications, fuel cells stand a good chance of replacing internal combustion engines at least. Before this happens, however, special infrastructure is necessary (the hydrogen needs to be stored somewhere, it requires special filling stations, pipes designed for high pressures, fuel tanks) and a number of improvements need to be made to fuel cells themselves.
Choosing the correct membrane is essential for improving fuel cells—the material that the membrane is made from must be as inexpensive as possible, chemically stable, technologically advanced, and its pores must provide adequate selectivity. Chemists and physicists are not simply going through materials at random, but conducting targeted experiments to create nanostructures with predetermined properties.
Molecular engineering
Scientists from MIPT, the Institute of Problems of Chemical Physics, Moscow State University, Institut de Sciences des Matériaux de Mulhouse, and DWI - Leibniz Institute for Interactive Materials of RWTH Aachen University have learned how to form pores from certain molecules for membranes of a fuel cell so that the opening is exactly the diameter required for the optimum functioning of the cell.
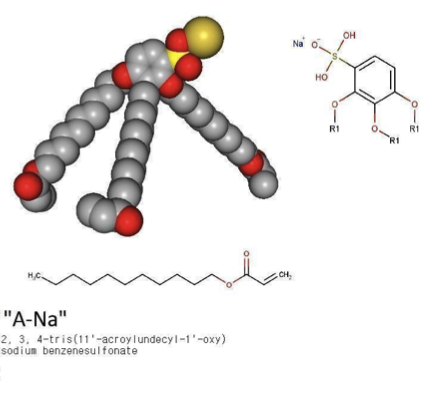
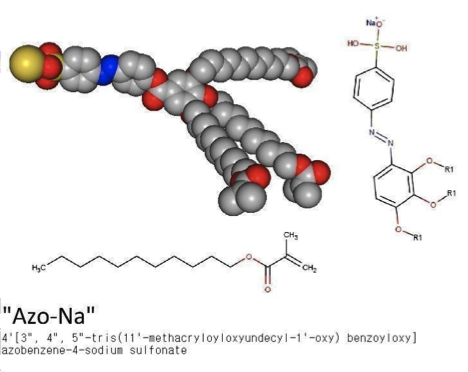
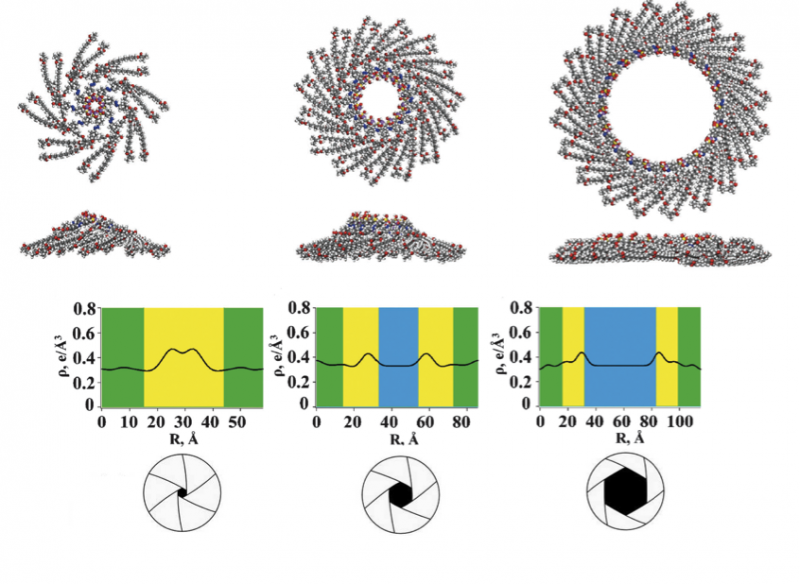
The molecules in question, A-Na and Azo-Na, are promising substances that are classified as benzenesulfonates. They are wedge-shaped (see image) and can independently assemble themselves into supramolecular structures—complex organized groups of multiple molecules. Depending on the conditions set by the scientists, the molecules form discs, which form columns with ion channels inside.
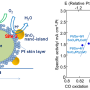
The pore size is directly related to the efficiency of the fuel cell. The selective permeability of these pores, which resemble the aperture of a camera, determines how efficiently ions are screened, and consequently how efficiently energy is converted in a fuel cell. Credit: authors of the study
This self-assembly of complex structures of individual molecules is possible due to their electrical properties. At one end of these molecules is a polar chemical group, i.e. a group with an electric charge, and in a solution, it naturally turns towards charged water molecules. At the other end of these molecules, there are non-polar hydrocarbon "tails" that, due to their electrical properties, try to stay as far away from water molecules as possible—this mechanism is what causes phenomena such as the formation of soap film, a cell membrane, and a fat droplet on the surface of cooking stock.
Scientists were able to predict the formation of these discs with pores and cylinders based on information on the structure of the benzenesulfonates being investigated, their geometry and physical and chemical properties. Using this information, the scientists first made a mathematical model based on the properties of complex supramolecular structures formed by A-Na and Azo-Na, and only then did they begin their experiments. During these experiments, they obtained various forms of ion channels, maintaining the substances at a certain humidity and temperature, and then irradiating them with UV light for polymerization.
The polymers created with this method were tested for selective permeability of ions and this enabled the scientists to identify which conditions of the synthesis of polymer membranes are best suited for making potential fuel cells.
From catalyst to molecules
The modern approach to producing structures that are ordered at a molecular level does not only involve computer models and logically choosing conditions to synthesize the required polymers. Researchers can now control the results of their work by directly observing the shape of the molecules or supramolecular structures they produce.
The structure of the complexes obtained was confirmed by X-ray scattering analysis of a synchrotron radiation source. This method is used when scientists need to find out the structure of something at a scale that cannot be seen with an optical microscope. The nanopores created by the researchers in their study were only a few nanometres wide; this is more than ten times smaller than a visible light wave.
At the European Synchrotron Radiation Facility in Grenoble (France) polymers were studied using X-ray analysis. X-rays were scattered over the samples and the analysis of the resulting diffraction pattern enabled the researchers to establish the exact size of the pores in the new polymers.
The pore size is directly related to the efficiency of the fuel cell. The selective permeability of these pores, which resemble the aperture of a camera, determines how efficiently ions are screened, and consequently how efficiently energy is converted in a fuel cell.
It converts chemical energy not into heat (as would be the case if hydrogen was burned in a burner), but into electricity. These devices were used on the Apollo lunar modules, and the Space Shuttle and Buran spacecraft systems. Credit: R.Dervisoglu / Wikimedia.
Global warming and molecular engineering
The new study, which MIPT specialists were actively involved in, does not only show how a promising material can be obtained from certain molecules and the methods that are used to do this. It allows us to look from an unexpected angle at a problem that at first glance may seem entirely unrelated to organic chemistry or X-ray analysis—the problem of climate change, a subject in the news recently after an international agreement was signed in Paris on the reduction of carbon emissions.
Today, it is almost unanimously recognized by the scientific community that the average temperature on the planet is rising and this is happening because of the increased concentration of carbon dioxide in the atmosphere. This gas, which traps heat, is mainly released by burning organic fuels. Therefore, an effective measure to prevent a rise in atmospheric carbon would be to switch to technologies that do not use oil, coal, and gas. However, radically redesigning virtually all technological infrastructure is not possible without an acceptable alternative to internal combustion engines, either electric accumulators and electric motors, or fuel cells with electric motors.
Fuel cells themselves, of course, will not solve the problem of atmospheric carbon. But they are part of a possible solution, and this means that self-organization of supramolecular structures from A-Na and Azo-Na can also be considered part of a global task. Even if it is not stated explicitly by the authors of a particular study, many scientific results can often influence people's lives in rather unexpected ways.
More information: K. N. Grafskaia et al. Designing the topology of ion nano-channels in the mesophases of amphiphilic wedge-shaped molecules, Phys. Chem. Chem. Phys. (2015). DOI: 10.1039/C5CP05618G
Journal information: Physical Chemistry Chemical Physics
Provided by Moscow Institute of Physics and Technology