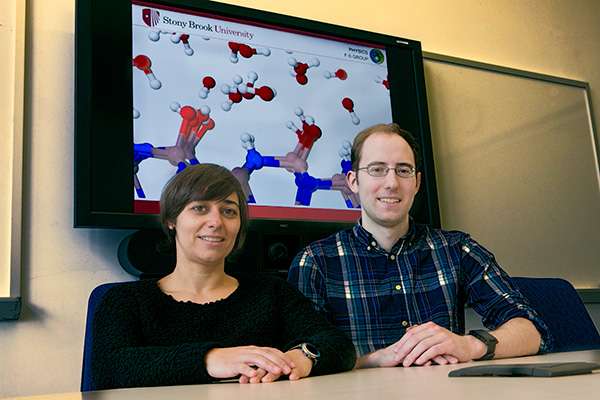
Daniel Elton and Marivi Fernandez-Serra used computer simulation models of water developed at Stony Brook’s Institute for Advanced Computational Science to discover its molecular properties are similar to ice.
For more than 100 years, scientists have debated what the underlying molecular structure of water is, and the common view has been that H2O molecules are either "water-like" or "ice-like." Now through computer simulation conducted at the Institute for Advanced Computational Science (IACS) at Stony Brook University, researchers can illustrate that the structure and dynamics of hydrogen bonding in liquid water is more similar to ice than previously thought. The finding, published in Nature Communications , changes the common understanding of the molecular nature of water and has relevance to many fields, such as climate science and molecular biophysics, and technologies such as desalinization and water-based energy production.
In condensed matter physics, phonons are considered to be a solid-state phenomenon and can be visualized as collective vibrations that propagate through a material. More precisely, a phonon is the fundamental quantum mechanical unit of lattice vibration. Optical phonons are a type of phonon that interact with electromagnetic radiation. These can be visualized as peaks in the infrared absorption spectrum in ice.
In the paper, "The hydrogen-bond network of water supports propagating optical phonon-like modes," lead author Daniel C. Elton, a PhD candidate, and Marivi Fernandez-Serra, PhD, Associate Professor, in the Department of Physics and Astronomy and IACS, show that propagating vibrations or phonons can exist in water, just as in ice.
"No microscopes can allow us to directly see the behavior of water molecules and their pattern of hydrogen bonding. Therefore by simulating liquid water using the fundamental laws of physics, the structure and motion of molecules in water can be analyzed in great detail beyond what microscopes can reveal of liquid water," said Elton. "Our method involved both experimental data and extensive molecular dynamics simulations, and we found that the optical phonon coupling leads to similar absorption peaks also found in ice."
The authors used a new high-powered computer cluster at Stony Brook's IACS to create the water dynamics simulations. By centering on water's unique hydrogen bond network, they routinely demonstrated that optical phonon-like modes can propagate the hydrogen bond network, just as in ice. Unlike in ice, however, hydrogen bonds in water are constantly being broken and reformed, so the phonons only last for about one trillionth of a second yet can travel over long distances up to two nanometers.
"Our findings challenge older ideas about water dynamics, which characterized peaks in the absorption spectrum as being due to the vibrational motions of at most a few molecules, as in ice," said Professor Fernandez-Serra. "We found water peaks in spectra correspond to two different types of phonons, called longitudinal and transverse. The shifting of the position of the longitudinal and transverse peaks with temperature can be related to important structural changes in the hydrogen bond network, which provides a new window into how water's structure changes with temperature."
Additionally, by comparing several different simulation techniques, the authors also found that the current non-polarizable water models used in biophysics fail to capture the higher frequency optical phonons. This work builds on their previous work , which showed that polarizable models are more accurate than the more often used non-polarizable models.
More information: Daniel C. Elton et al. The hydrogen-bond network of water supports propagating optical phonon-like modes, Nature Communications (2016). DOI: 10.1038/ncomms10193
D. C. Elton et al. Polar nanoregions in water: A study of the dielectric properties of TIP4P/2005, TIP4P/2005f and TTM3F, The Journal of Chemical Physics (2014). DOI: 10.1063/1.4869110
Daniel C. Elton's blog: www.danielcelton.com/2016/01/1 … ons-in-liquid-water/
Journal information: Nature Communications , Journal of Chemical Physics
Provided by Stony Brook University