In aging societies, atherosclerosis (AS) and its major vascular complications, myocardial infarction and stroke, comprise a leading cause of morbidity and mortality. Additionally, childhood obesity is an increasing phenomenon all over the globe and will lead to increased occurrence of cardiovascular diseases at younger age. Research toward non-invasive methods for disease diagnosis is particularly important for these patients in order to reduce the risk of invasive analysis and X-ray radiation.
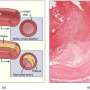
Plaques consist of fatty substances and leukocytes and accumulate at the walls of arteries, making them thicker. There are two different plaque types: stable and vulnerable plaques. Stable AS plaques tend to be rich in extracellular matrices and smooth muscle cells. They may be asymptomatic for many years, until lumen stenosis is severe. Unstable plaques are rich in certain macrophage subsets, foam cells and inflammatory cells, and usually have a weak fibrous cap. They are prone to rupture into the circulation, inducing thrombus formation in the lumen—one of the most common and fatal complications of AS. The consequences may be a myocardial infarction, an ischemic stroke, or a claudication from lack of blood supply to the legs. These vulnerable plaques represent a high risk, particularly with the standard invasive diagnosis by coronary angiography. Yet so far, there are no non-invasive, low-risk clinical approaches available to detect and distinguish AS plaque types in vivo.
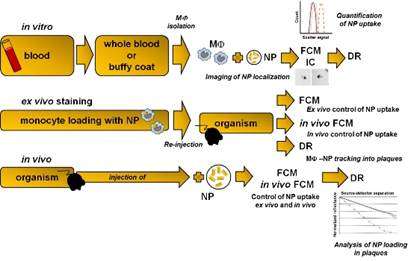
Scientists from the University of Leipzig, the Translational Centre for Regenerative Medicine (TRM) Leipzig (Germany), and the Bar-Ilan University (Israel) now suggest a novel, highly sensitive, simple, inexpensive and non-invasive biophotonics method that could lead to identification of unstable plaques. The new approach is based on the presence of macrophages in vulnerable plaques and their ability to take up gold nanoparticles (GNP). Cells loaded with GNP could be detected by the diffusion reflection method in AS plaques.
There are several possible ways how the approach could be put into clinical practice. One could be the administration of GNP by intravenous injection. GNP then should be specifically phagocytized by tissue macrophages or by circulating monocytes/macrophages that infiltrate tissues. Due to their physical properties, phagocytized GNP induce changes in the optical characteristics of infiltrated tissues that could be efficiently visualized by diffusion reflection (DR) using infrared light.
For quality assurance, an approach for monocyte subtype-selective GNP loading and quantification of the differential particle loading efficiency using cytometry was developed, as well.
More information: Susanne Melzer et al. Nanoparticle uptake by macrophages in vulnerable plaques for atherosclerosis diagnosis, Journal of Biophotonics (2015). DOI: 10.1002/jbio.201500114
Provided by Wiley