Researchers have observed how a light-activated compound alters the structure of DNA - which could be the first step in creating new, targeted cancer treatments.
Photo-dynamic therapy, a form of treatment for a number of conditions including several cancers and psoriasis, is an area of intense research activity. This treatment strategy uses light to activate a drug in a specific area of the body and can reduce the side effects observed through conventional anti-cancer treatments.
Now a group of scientists working in Reading and Dublin have published a new way of finding out how such compounds work at a fundamental level, the information from which can be used to improve anti-cancer drug development.
It is really difficult to observe such fast processes in living cells, but the much simpler environment of a DNA crystal has enabled the team to watch the initial crucial step in great detail. The new method has been published this week in the leading chemical journal Nature Chemistry.
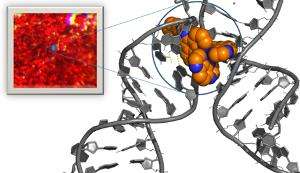
The crystals contain a ruthenium compound which is bound to a short piece of DNA. This class of compound is used in DNA sensing and is of interest to the pharmaceutical industry for cancer treatment.
Fast process
The researchers found that by using infrared radiation, they could get a snapshot of the extremely fast process - which occurs in half a billionth of a second - that takes place when light is shone on the crystals. This activates the compound, making it cause damage to DNA.
This research was carried out using two UK national research facilities: the Science and Technology Facilities Council's (STFC) Central Laser Facility and the Diamond Light Source, the UK national synchrotron.
Dr Susan Quinn, from the School of Chemistry at University College Dublin is the lead author of the study. She said: "These results are very exciting as they demonstrate the ability to follow the flow of electrons from DNA to a molecule whose exact position is known and this is an enormous advantage in the study of the early events that lead to DNA damage."
Professor Christine Cardin, from the University of Reading, is a nucleic acid crystallographer who led the UK team, including co-author Dr James Hall, and who has received major funding from BBSRC in support of this work.
She said: "This work is an exciting step in helping us to understand DNA damage. Among other things, the insights from this study will feed into the development of new drugs that target cancerous tissue, without damaging healthy tissue around it.
"This paper is one result of a longstanding collaboration with Professor John Kelly in Trinity College Dublin, and an excellent example of multidisciplinary international collaboration.
"It also highlights the benefits of combining the facilities of Diamond Light Source, where all the crystallography has been carried out, with the equipment and expertise available in the adjacent Central Laser Facility of the STFC."
Dr Mike Towrie from STFC's Central Laser Facility said: "Metal complexes that bind to DNA are now used in chemotherapies for cancer, or have potential activity against drug resistant bacteria.
"Ruthenium complexes are of particular interest because they bind reversibly into the grooves of DNA, have a degree of DNA site selectivity and absorb light in the visible spectral region so might be applicable in photodynamic therapies. They have the added benefit that they have 'light switch' properties that tell you when they are attached to DNA in the cell because they become luminescent, which is useful as a biological probe for both living and inactive cell studies."
More information: James P. Hall et al. Monitoring one-electron photo-oxidation of guanine in DNA crystals using ultrafast infrared spectroscopy, Nature Chemistry (2015). DOI: 10.1038/nchem.2369
Journal information: Nature Chemistry
Provided by University of Reading