Nanocarbons clean water without the use of chemicals and transform waste heat into electricity

When purifying waste water, chemical reactions are used or it is subjected to elaborate filtering methods to remove salts and heavy metals. Now scientists in Saarbrücken are working on a desalination method which does not require the addition of chemicals and which is highly energy-efficient: in the process known as capacitive de-ionization (CDI), electrodes are used to extract the ions from the water and collect them on the electrodes. The result is clean water and ions which have been enriched on the electrodes. This environmentally friendly process can even be used to generate electricity: emissions such as carbon dioxide are also suited to generating electrical energy when they are dissolved in water as ions.
At an international conference in Saarbrücken, more than 110 experts from 20 countries will be exchanging their knowledge from 26th to 28th October in order to further understand and improve the materials and processes which form the basis of CDI. The conference is being organized on the Saarbrücken Campus by the INM – Leibniz Institute for New Materials.
The principle of CDI not only serves the purpose of removing unwanted ions from the water. Volker Presser, head of the Energy Materials Group at INM explains, "The generation of electricity can also be the main desired effect, to use emissions from power plants to produce electricity, for example". For this purpose, the emissions merely has to be present in the water as ions. "Carbon dioxide, for example, is very well suited to this purpose" Professor Presser said and added: "It is particularly exciting for us that, thanks to the electrosorption process, we can even convert waste heat into electricity". He said this worked because the electrodes are charged at low temperatures and discharged at higher temperatures. As a result of the temperature increase, the electrical voltage increases so that electrical energy can be recovered directly during discharging.
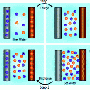
The basic principle of CDI functions without chemical reactions thanks to ion-electrosorption: the water to be purified flows between two electrodes made of porous carbon to which a voltage is applied. The positively charged electrode extracts the ions which have a negative charge from the water and the negatively charged electrode, located opposite, extracts the ions with a positive charge from the water. The ions are stored in the nanopores of the electrode material and, at the end of the process, clean water flows out.
"To achieve the highest possible degree of efficiency, it is not sufficient to simply use 'any' porous activated carbon as electrode material", Presser, the young Saarbrücken-based researcher commented. One focus of his work and an important topic of discussion at the conference is therefore the synthesis and characterisation of new carbon-nanomaterials such as graphenes or so-called carbon nano-onions.
Die CDI&E International Conference on Capacitive Deionization and Electrosorption will take place from 26th to 28th October in the auditorium of the campus of the Saarland University. The conference is chaired by the German-Israeli team consisting of Professor Volker Presser and Professor Matthew Suss from Technion – the Israel Institute of Technology.
A large number of participants come from Asian and Arab regions since countries where there is a marked scarcity of water are doing intensive research into the technology. CDI is also an attractive option for processing water from mine workings: not only do you get clean water; the ions or precious metals retained as a highly enriched fluid can be a valuable resource in industry.
In ten programme units with supplementary posters, scientists will discuss their results and findings regarding possible materials and mechanisms of electrodes in CDI, energy generation, extraction of ions and ionic processes. Professor emeritus Bertel Kastening, a well-known pioneer of electro-chemistry, has been invited as guest of honour.
Provided by Leibniz Institute for New Materials