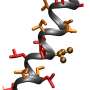
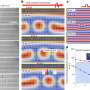
SDSU investigators found that flies with a double-mutation in their myosin protein had better protein function than those with a single mutation. Credit: Mr. checker/Wikimedia Commons
Two wrongs don't make a right, but in the case of genetic mutations, having two mutations in the same gene could be better than having either one individually. Recent research by biologists at San Diego State University found that two separate genetic modifications each greatly reduced the function of the myosin muscle protein in fruit flies, but flies with both mutations had nearly three-quarters of the protein function restored. The findings are important for researchers looking to better understand and treat heart muscle disease in humans.
Myosin is a motor protein involved in muscle contraction. The proper functioning of this protein depends on a series of chemical bonds that hold the protein in its proper configuration. Mutating the protein to destroy some of these bonds can cause the protein to lose some or all of its function, leading to abnormal muscle contraction in the organism.
Earlier studies had suggested that within the myosin protein, the interaction between two amino acid subunits called E497 and R712 is charge-dependent. In other words, in order to normally function, a negative charge on one amino acid interacts with a positive charge on the other. In humans, mutations in either of these amino acids can result in hypertrophic cardiomyopathy, a common form of inherited heart muscle disease. In a recent study, investigators at SDSU looked further into how exactly disrupting the charges of these amino acids might lead to muscle defects.
Mutant muscles
SDSU biologist Sanford Bernstein and colleagues experimented with fruit flies with genetically modified proteins in the flies' flight muscles to see what effects that would have on the proteins' functioning. The researchers bred two versions of fruit flies in which either the fly version of the E497 or the R712 myosin protein amino acids were mutated by having their charges reversed. Those flies lost their flight ability completely. Using a combination of biochemical techniques, the researchers found that the myosin proteins were about five times less active in these flies.
A third line of genetically modified flies had the charges reversed in both E497 and R712 myosin protein amino acids. These flies still couldn't fly, but the researchers found that the proteins were about 73-percent as active as those in normal, unmodified flies—a vast improvement over having either single mutation by itself.
Interestingly, heterozygous flies (those with copies of both normally functioning and genetically modified genes) in the study did manage to fly when they were young. However, this occurred only for the double-mutant. Overall, the double-mutation restored most of the protein activity that was lost in the other two mutant lines, demonstrating that both amino acids need to interact in a charge-dependent manner in order for normal muscle protein function to occur. The researchers published their results earlier this month in the Journal of Biological Chemistry.
Helping humans
Because the myosin protein modified in the flies is so similar to the one known to be involved in some human cardiomyopathy, and since the same amino acids are affected, Bernstein said that a similarly dysfunctional process probably underlies the disease caused by mutations in these specific amino acids in humans.
"In humans, it's a different type of mutation, but it's one that we predict is disruptive to normal interaction within the protein, resulting in decreased or possibly increased myosin function," he said.
For that reason, researchers can further study these proteins in flies to learn more about the disease. Bernstein said that the team's results suggest that the abnormal function in human heart muscle cells is largely a result of structural failings in the myosin protein that lead to biochemical dysfunction and an unsuccessful attempt to compensate for these defects, resulting in abnormal heart structure and function.
What's more, the findings point to the potential for drugs that can modify myosin function to overcome connection errors within abnormally charged myosin proteins, which might improve functioning in human heart muscle cells.
More information: William A. Kronert et al. A Failure to Communicate: Myosin Residues Involved in Hypertrophic Cardiomyopathy Affect Inter-Domain Interaction, Journal of Biological Chemistry (2015). DOI: 10.1074/jbc.M115.681874
Journal information: Journal of Biological Chemistry
Provided by San Diego State University