From good to bad with a copper switch
At the molecular level, the difference between Doctor Jekyll and Mr Hyde lies in a metal, copper. In its physiological form, the prion protein (PrPC ) is 'good' and is involved in normal body processes. It can happen, however, that because of some as yet unknown mechanism, it changes form and turns into a threat for the health of humans and animals (it is responsible for neurodegenerative diseases such as spongiform encephalopathies). According to a new SISSA study, the mechanism underlying this change is a metal, copper, or rather a particular region of the protein to which the metal binds, which acts as a sort of 'switch' that turns PrPC into its terrible alter ego.
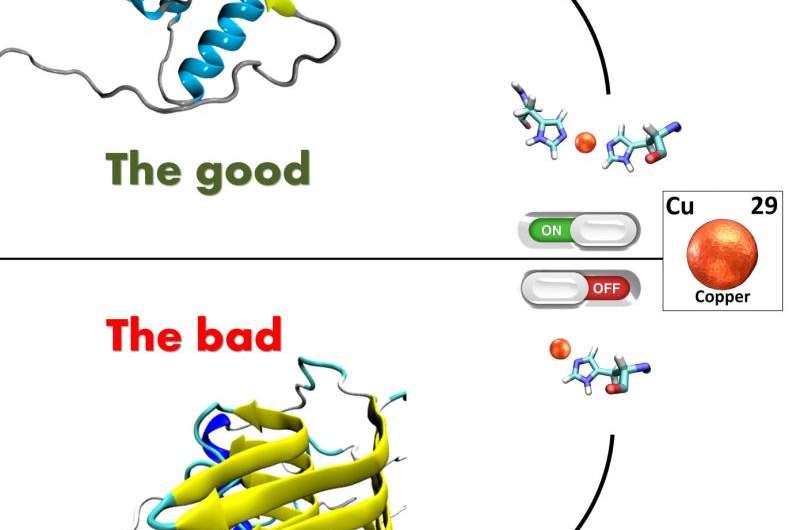
"We still don't know what complex molecular mechanisms cause the prion protein to become bad," explains Giuseppe Legname, professor at the International School for Advanced Studies (SISSA) in Trieste who coordinated the new study, "nor do we know any treatments to cure prion diseases. Our research has finally uncovered a critical cofactor, which is capable of triggering the transformation of prions proteins from good to bad. And this cofactor is copper which binds to an amino acid sequence of the prion protein, known as 'fifth copper binding site', which has so far been poorly studied".
"In physiological conditions, copper is tightly bound to two histidine amino acids", continues Legname. "When copper is bound in this way it seems to protect the prion protein. When instead copper is missing or is bound to one rather than two histidines, that's when problems arise: the prion protein becomes unstable and turns into a bad and infectious prion".
To reach this conclusion, the researchers used multidisciplinary experimental approaches, ranging from structural to cellular biology. "It all started with an intuition we published in the journal Biochemistry in 2012", explains Gabriele Giachin, first author of the study and former SISSA PhD student (today at the European Synchrotron Radiation Facility, ESRF, in Grenoble, France). "On that occasion, we hypothesized that the pathological genetic mutations present in the prion protein could affect copper coordination". Starting from this intuition, Giachin and colleagues went on to conduct in-depth experiments using XAFS (X-ray absorption fine structure) spectroscopy, exploiting the powerful X-rays available at the Grenoble synchrotron. Then, drawing on the consolidated expertise in molecular and cellular biology available at the SISSA Laboratory of Prion Biology coordinated by Legname, the group confirmed the hypothesis in living cell systems.
"These results finally answer a fundamental question: what mechanism underlies the appearance of prions?", concludes Legname. "We have been the first to provide a detailed description of the role of copper in prion conversion, opening the way for the development of new drugs targeting this copper binding site, and thus for new potential treatments".
Journal information: Biochemistry
Provided by International School of Advanced Studies (SISSA)