With the growth of wind and solar energy and the increasing popularity of electric vehicles, many people in the U.S. may have forgotten about the promised "hydrogen economy." But in research labs around the world, progress continues. Now scientists are reporting in the Journal of the American Chemical Society a new process that could help us move faster toward sustainable hydrogen-based energy.
One of the major challenges to developing affordable hydrogen fuel cells has been storage. Hydrogen is explosive and requires costly containers to hold it safely. But recently, scientists have shown that formic acid is a good candidate for storing hydrogen. The common industrial chemical—also the stuff of ant venom—is stable and inexpensive. One molecule of the acid is made of five atoms, two of which are hydrogen atoms. But splitting the formic acid to release hydrogen and produce electricity requires a lot of heating and processing. So Qiang Xu and colleagues set out to find a better way.
The researchers developed a simple method for producing a palladium-based nanomaterial that can spur the breakdown of formic acid into hydrogen and carbon dioxide. Its efficiency far exceeded that of any other reported heterogeneous catalysts, they say. They also found that their process only produced carbon dioxide and hydrogen without carbon monoxide contamination, which has been a problem with other methods.
More information: Immobilizing Extremely Catalytically Active Palladium Nanoparticles to Carbon Nanospheres: A Weakly-Capping Growth Approach, J. Am. Chem. Soc., 2015, 137 (36), pp 11743–11748, DOI: 10.1021/jacs.5b06707
Abstract
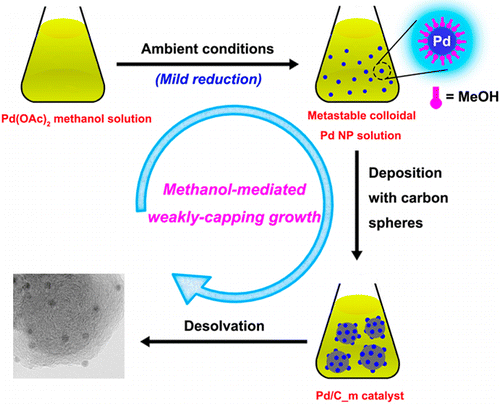
Ultrafine palladium nanoparticles (Pd NPs) supported on carbon nanospheres have been successfully synthesized using a facile methanol-mediated weakly-capping growth approach (WCGA) with anhydrous methanol as a mild reductant and a weakly capping agent. The Pd NPs show exceedingly high catalytic activity for 100% selective dehydrogenation of aqueous formic acid (FA) at ambient temperatures. The small size and clean surface of the Pd NPs greatly improve the catalytic properties of the as-prepared catalyst, providing an average rate of CO-free H2 generation up to 43 L H2 gPd–1 min–1 and a turnover frequency of 7256 h–1 at 60 °C. These values are much higher than those obtained even with the most active catalyst reported thus far for heterogeneously catalyzed dehydrogenation of FA. This remarkably facile and effective methanol-mediated WCGA provides a powerful entry into ultrafine metal NPs with clean surface to achieve enhanced performance. Moreover, the catalytic results open up new avenues in the effective applications of FA for hydrogen storage.
Journal information: Journal of the American Chemical Society
Provided by American Chemical Society