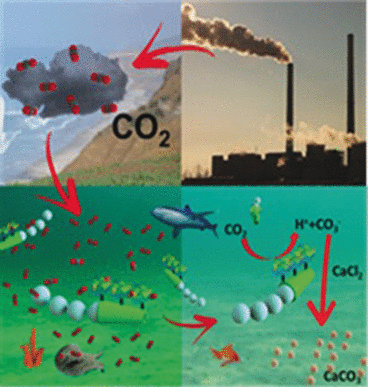
Rapid decontamination of an aqueous solution by a freely moving microscrubber: this scenario has been realized by American scientists for the sequestration of CO2 from water. In the journal Angewandte Chemie, they introduce their concept of enzymatic conversion of CO2 into solid calcium carbonate, which is greatly facilitated by the use of self-propelled micromotors that act as a movable enzyme support.
The on-site mineralization of the gaseous CO2 into solid and durable carbonate salts is one of the options that scientists consider feasible for tackling the issue of the ongoing and massive man-made release of carbon dioxide by the combustion of fossil fuels. Calcium carbonate is one of the preferred storage forms for CO2, and marine organisms have piled up layers of calcium carbonate as thick as mountains by bioconversion of carbon dioxide over millions of years, However, the uncatalyzed formation of carbonates from carbon dioxide in aqueous environments is too slow to be practically applicable for large-scale CO2 sequestration by man. Joseph Wang and his group at the University of California, San Diego, have now greatly speeded up this conversion by a cunning chemical-nanoengineering approach. "Our approach combines the biocatalytic activity of carbonic anhydrase [a zinc metalloenzyme that catalyzes the hydration of CO2 to form bicarbonate] with the self-propulsion of chemically powered micromotors through CO2-saturated samples to act as highly efficient mobile biocatalytic microscrubbers," they write. The main advantage of this method is the automatic self-mixing and scrubbing of the reaction solution just by adding environmentally friendly hydrogen peroxide, the "fuel" of the micromotors.
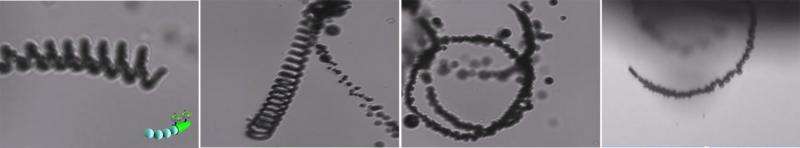
As the micromotors, the scientists used a six micrometer sized tube made of a modified polymer. At its inner surface, the hydrogen peroxide is catalytically converted into water and oxygen gas. It is the thrust of the oxygen gas bubbles that pushes the microtube into a forward movement. At the outer surface of the tube the scientists anchored the carbonic anhydrase enzyme. Fueled by hydrogen peroxide, this enzyme-micromotor combination propels through the aqueous solution like a submarine, reaching the impressive speed of more than 100 micrometers per second, or eighteen times its length, the authors write. But the speed is not the only advantage of the submarine microscrubber, the chemical anchoring of the enzyme on the micromotor surface also grants the enzyme's stability for CO2 hydration.
These are video frames showing the movement of a micromotor in sea water. Credit: Laboratory for Nanobioelectronics, UC San Diego Jacobs School of Engineering
Tested in action, the microscrubber decontaminated the CO2-containing test solutions, including seawater, efficiently within several minutes. Which sounds like a man-made attempt to pile up calcium carbonate rocks again.
More information: "Micromotor-Based Biomimetic Carbon Dioxide Sequestration: Towards Mobile Microscrubbers." Angew. Chem. Int. Ed.. DOI: 10.1002/anie.201505155
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Angewandte Chemie