Team genetically engineers yeast to produce opioids
For thousands of years, people have used yeast to ferment wine, brew beer and leaven bread.
Now researchers at Stanford have genetically engineered yeast to make painkilling medicines, a breakthrough that heralds a faster and potentially less expensive way to produce many different types of plant-based medicines.
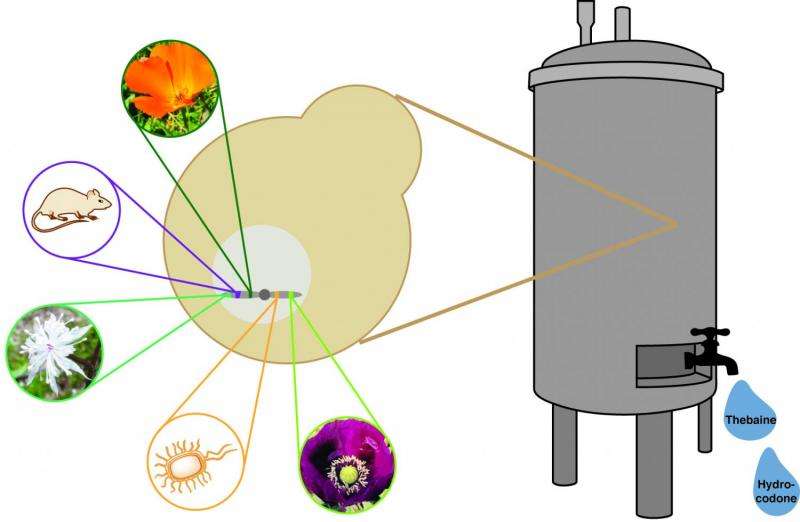
Writing today in Science, the Stanford engineers describe how they reprogrammed the genetic machinery of baker's yeast so that these fast-growing cells could convert sugar into hydrocodone in just three to five days.
Hydrocodone and its chemical relatives such as morphine and oxycodone are opioids, members of a family of painkilling drugs sourced from the opium poppy. It can take more than a year to produce a batch of medicine, starting from the farms in Australia, Europe and elsewhere that are licensed to grow opium poppies. Plant material must then be harvested, processed and shipped to pharmaceutical factories in the United States, where the active drug molecules are extracted and refined into medicines.
"When we started work a decade ago, many experts thought it would be impossible to engineer yeast to replace the entire farm-to-factory process," said senior author Christina Smolke, an associate professor of bioengineering at Stanford.
Now, though the output is small - it would take 4,400 gallons of bioengineered yeast to produce a single dose of pain relief - the experiment proves that bioengineered yeast can make complex plant-based medicines.
"This is only the beginning," Smolke said. "The techniques we developed and demonstrate for opioid pain relievers can be adapted to produce many plant-derived compounds to fight cancers, infectious diseases and chronic conditions such as high blood pressure and arthritis."
From plant to test tubes
Many medicines are derived from plants, which our ancestors chewed or brewed into teas, or later refined into pills using chemical processes to extract and concentrate their active ingredients. Smolke's team is modernizing the process by inserting precisely engineered snippets of DNA into cells, such as yeast, to reprogram the cells into custom chemical assembly lines to produce medicinal compounds.
An important predecessor to the Stanford work has been the use of genetically engineered yeast to produce the anti-malarial drug artemisinin. Traditionally artemisinin has been sourced from the sweet wormwood tree in similar fashion to how opiates are refined from poppy. Over the last decade, as yeast-based artemisinin production has become possible, about one third of the world's supply has shifted to bioreactors.
The artemisinin experiments proved that yeast biosynthesis was possible, but involved adding only six genes. The Stanford team had to engineer 23 genes into yeast to create their cellular assembly line for hydrocodone.
"This is the most complicated chemical synthesis ever engineered in yeast," Smolke said.
Her team found and fine-tuned snippets of DNA from other plants, bacteria and even rats. These genes equipped the yeast to produce all the enzymes necessary for the cells to convert sugar into hydrocodone, a compound that deactivates pain receptors in the brain.
"Enzymes make and break molecules," said Stephanie Galanie, a PhD student in chemistry and a member of Smolke's team. "They're the action heroes of biology."
To get the yeast assembly line going, the Stanford team had to fill in a missing link in the basic science of plant-based medicines.
Many plants, including opium poppies, produce (S)-reticuline, a molecule that is a precursor to active ingredients with medicinal properties. In the opium poppy, (S)-reticuline is naturally reconfigured into a variant called (R)-reticuline, a molecule that starts the plant down a path toward the production of molecules that can relieve pain.
Smolke's team and two other labs recently independently discovered which enzyme reconfigures reticuline, but even after the Stanford bioengineers added this enzyme into their microbial factory, the yeast didn't create enough of the opioid compound. So they genetically tweaked the next enzyme in the process to boost production. Down the line they went, adding enzymes, including six from rats, in order to craft a molecule that emerged ready to plug pain receptors in the brain.

Engineered with a purpose
In their Science paper, the Stanford authors acknowledged that a new process to make opioid painkillers could increase concerns about the potential for opioid abuse.
"We want there to be an open deliberative process to bring researchers and policymakers together," Smolke said. "We need options to help ensure that the bio-based production of medicinal compounds is developed in the most responsible way."
Smolke said that in the United States, where opioid medicines are already widely available, the focus is on potential misuse. But the World Health Organization estimates that 5.5 billion people have little or no access to pain medications.
"Biotech production could lower costs and, with proper controls against abuse, allow bioreactors to be located where they are needed," she said.
In addition to bioengineering yeast to convert sugar into hydrocodone, the Stanford team developed a second strain that can process sugar into thebaine, a precursor to other opioid compounds. Bio-produced thebaine would still need to be refined through sophisticated processes in pharmaceutical factories, but it would eliminate the time delay of growing poppies.
"The molecules we produced and the techniques we developed show that it is possible to make important medicines from scratch using only yeast," she said. "If responsibly developed, we can make and fairly provide medicines to all who need."
More information: "Complete biosynthesis of opioids in yeast," by S. Galanie et al. www.sciencemag.org/lookup/doi/ … 1126/science.aac9373
Journal information: Science
Provided by Stanford University