Scientists track ultrafast formation of catalyst with X-ray laser
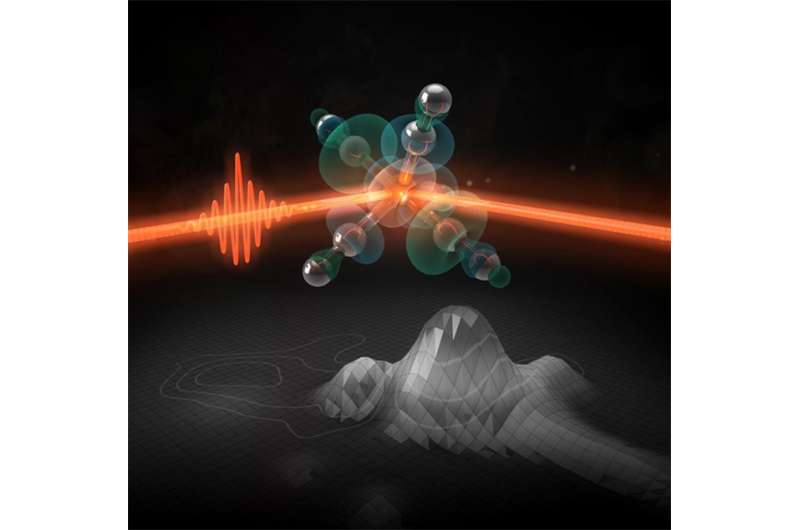
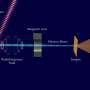
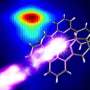
At the SLAC National Accelerator Laboratory, an international team has – for the first time – precisely tracked the surprisingly rapid process by which light rearranges the outermost electrons of a metal compound known as Fe(CO)5 and turns it into an active catalyst – a substance that promotes chemical reactions. The results come from using resonant inelastic x-ray scattering at SLAC, which also provides information about the chemical dynamics of the catalyst.
The new x-ray spectroscopy technique allows scientists to investigate how light rearranges the outermost electrons of a compound in a few hundred femtoseconds, or quadrillionths of a second. Learning these details could help scientists predict and control the quick, early steps in reactions to turn sunlight and water into fuel as well as master the chemistry required to produce other renewable fuels.
The proper methods to interrogate the electronic states of transition metal complexes, such as the iron-based Fe(CO)5, during a reaction have been lacking. Recently, a team of researchers from the United States and Europe employed a technique designed to alleviate this problem. This research group used resonant inelastic x-ray scattering (RIXS) at the SLAC National Accelerator Laboratory to probe the frontier or outermost orbitals of Fe(CO)5 with atomic-level and femtosecond resolution. In this work, the researchers used femtosecond duration x-ray laser pulses generated by the Linac Coherent Light Source to follow Fe-CO bond dissociation in the metal complex and the resulting catalyst formation.
The measurement is the first of its kind, showing the potential for using multi-dimensional x-ray spectroscopy to probe chemical dynamics and better understand how catalysts function. Data acquired through this technique has already addressed a long-standing question in the photochemistry of the catalyst Fe(CO)4 by demonstrating that triplet and singlet reaction pathways occur in parallel even on the sub-picosecond timescale. The use of RIXS should be applicable to a variety of research fields, especially those looking to gain information on structural dynamics in ultrafast processes.
More information: "Orbital-specific mapping of the ligand exchange dynamics of Fe(CO)5 in solution." Nature 520, 78-81 (2015). DOI: 10.1038/nature14296
Journal information: Nature
Provided by US Department of Energy