The looming fossil energy crisis and serious environment and climate issues urgently call for sustainable energy systems and next-generation energy storage technologies. Instead of a traditional "carbon cycle" based on fossil energy, the "hydrogen cycle" has emerged and may be a promising alternative. With a water splitting device, H2 can be generated from water by electricity or solar energy, and energy transforms between electrical/solar and chemical energy in rechargeable batteries. However, the core issue of water splitting, oxygen evolution reaction (OER) (4OH- —> 2H2O + O2 + 4e-, in base), is a kinetically sluggish half-reaction, which requires a high overpotential and hinders the development of water splitting.
Recently, a research group from China, led by Prof. Qiang Zhang in Tsinghua University, has developed a novel graphene/metal hydroxide composite with superior oxygen evolution activity. This work is published in the journal Advanced Materials.
On one hand, graphene is a material that exhibits ultrahigh electrical conductivity, high surface area, and tunable 3D structures, which is excellent for heterogeneous electrocatalysis. However, the intrinsic activity of graphene is undesirable. On the other hand, NiFe layered double hydroxides (NiFe LDHs), with remarkable catalytic activity, high stability, earth-abundant and environmental benign characters, are regarded as the most promising nonprecious metal catalysts.
"Therefore, the fine control of NiFe LDH hybridization into a specific graphene substrate to obtain an increased electrochemical active surface area (ECSA), fully exposed active sites, and an optimal interfacial junction is the most promising recent topic towards superior oxygen evolution catalysis and practical application," Prof. Qiang Zhang says.
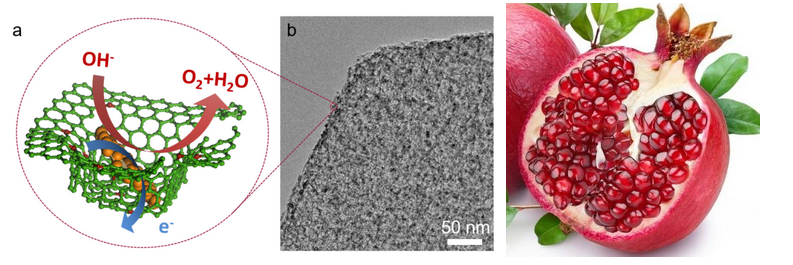
In this work, the architecture of graphene/NiFe LDH composites is inspired by the hierarchical structure of pomegranate. By using a nitrogen-doped mesoporous graphene framework as the substrate for the in situ growth and decoration of NiFe LDHs, the resultant LDHs exhibit a uniform nano-size and dispersion, and a strong interfacial couple with the conductive substrate.
"The most important issue for the material fabrication is the topology-assisted and spatially-confined growth strategy due to the graphene." says Cheng Tang, the first author of this work. "The nitrogen dopant and topology-induced defects of graphene contribute to the adsorption and anchor of metal cations and then the in-plane mesopores on graphene serve as nano-reactors for spatially-confined nucleation and growth of NiFe LDHs, thereby rendering a strong affinity and uniform dispersion of the as-grown nanosized NiFe LDH in the mesoporous graphene framework."
"This hierarchical structure optimizes the hybridization between NiFe LDHs and graphene," Prof. Zhang notes. "It results in mesoporous channels, interconnected electron freeway, intimate interfacial coupling, repressed particle aggregation, and fully exposed active sites."
Further catalysis measurements reveal that this material overperforms commercial Ir/C catalysts and competes favorably against the best reported alternatives for high-performance OER catalysis with a remarkably low Tafel slope (~45 mV dec-1), a substantially decreased overpotential (~337 mV required for 10 mA cm-2), and enhanced durability in 0.10 M KOH.
Prof. Zhang and his team report that the excellent performance is contributed from the synergetic effect of two ideal components and also the unique structure features of this novel hybrid. Going forward, they plan to investigate and optimize the composition and structure of this kind of hybrid, and to ascertain the structure-property relationships and underlying catalytic mechanism.
"I believe that this strongly-coupled complex has various applications such as heterogeneous catalysis, sensors, energy conversion and storage, and so o,." says Prof. Zhang. "And more importantly, the topology-assisted design and fabrication strategy opens up new avenues and sheds light on a novel branch of advanced nano-architectured materials and hybrids."
More information: "Spatially Confined Hybridization of Nanometer-Sized NiFe Hydroxides into Nitrogen-Doped Graphene Frameworks Leading to Superior Oxygen Evolution Reactivity." Advanced Materials 2015, DOI: 10.1002/adma.201501901.
Journal information: Advanced Materials
Provided by Tsinghua University