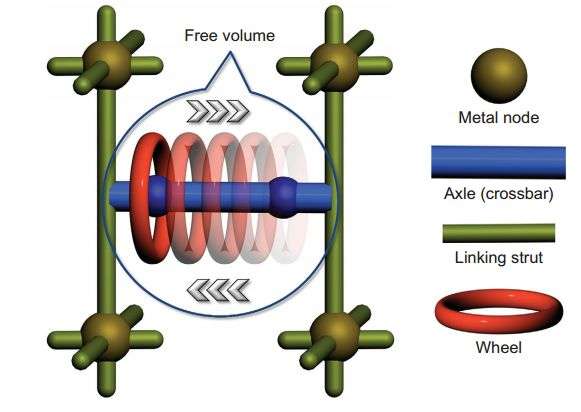
MOF materials are commonly constructed from a combination of rigid linking struts (green) and metal nodes (brown). Credit: Kelong Zhu et al. Nature Chemistry, 2015: doi:10.1038/nchem.2258
(Phys.org)—In 1959 Moore observed that from the time the integrated circuit was invented, the number of transistors per square inch doubled about every eighteen months. A contemporary of Moore, Feynman, suggested that denser circuitry could be achieved by making molecular-scale circuitry. Since that time, mechanically interlocked molecules (MIMs) have proven a viable contender for eventually making molecular-based circuitry, including molecular switches. However, most molecular switches are made and studied in solution.
Kelong Zhu, Christopher A. O'Keefe, V. Nicholas Vukotic, Robert W. Schurko and Stephen J. Loeb from the Department of Chemistry and Biochemistry at the University of Windsor have designed and characterized a molecular shuttle that functions both in solution and when placed within a rigid chemical structure called a metal-organic framework. Their work appears in Nature Chemistry.
This research makes use of the rotaxane architecture, a MIM comprised of a ring-shaped molecule and two recognition sites. Rotaxanes have two components: A molecule is threaded through a macrocyclic ring, like a wheel with an axle. The macrocycle moves linearly along the axle between two recognition sites. Zhu, et al. used a 24-crown-8 macrocycle and benzimidazole recognition sites on the axle.
The authors used a metal-organic framework (MOF) as a rigid structure in which they can make the axle of the [2]rotaxane between struts on the MOF. MOFs provide synthetic versatility while maintaining structural integrity in a diversity of environments.
Their prior research indicated that they could control the movement of the molecular shuttle using heat without disturbing the MOF and [2]rotaxane architecture. Zhu, et al. began by constructing and testing their molecular shuttle in solution to determine the translational energy barrier as well as the translation rate. They, then, made the same [2]rotaxane within a Zn(II)-based MOF and compared its translational energy and rate to the system in solution.
For solution studies, they took a 13C labeled compound and studied its translational rate with respect to temperature using variable temperature 13C NMR. Results showed that the energy barrier for molecular shuttling was 7.7 kcal mol-1 and the rate was 1.4 x 107 s-1 at 298 K.
The next step was to construct the MOF-[2]rotaxane system to see if the molecular shuttle worked in solid-state conditions. They took the MIM used for solution studies and combined it with Zn(II) tetrafluoroborate hydrate using a known procedure for making a Zn(II)-based MOF, and obtained yellow crystals of product in excellent yields.
One necessary step after the synthesis was to neutralize one of the benzimidazole recognitions sites. During synthesis, one site obtained a +1 charge while the other did not. 24-crown-8 coordinates preferentially to a positively charged species, meaning that it would remain on the positively charged site and never traverse to the neutral recognition site. Zhu, et al. devised a way to neutralize the benzimidazole linker without damaging the structural integrity of the MOF-[2]rotaxane. The neutralized final product was labeled UWDM-4.
The structure, based on X-ray analysis, indicates that the structure has a Zn4O cluster. Carboxylate groups coordinate to this cluster, triphenyl struts form the sides of the cubic structure, and the benzimidazole recognition sites along the [2]rotaxane axle connect the cubes.
Finally, the UWDM-4 shuttling mechanism was tested in the same way that the shuttle in solution was tested. UWDM-4 was 13C enriched at the recognition sites and tested using variable temperature 13C solid-state NMR. Results revealed that the shuttle worked in this confined structure, although the translational energy barrier and rate are different from the solutions studies. The energy barrier for UWDM-4 was 14.1 kcal mol-1 and the rate was 283 s-1 at 298 K.
The authors speculate that the difference between the solid and liquid states have to do with enthalpic and entropic effects that may be due to steric hindrance and molecular conformation restrictions in the confined solid system. Additionally, solvent effects likely play a role in the energy and rate differences.
This research shows that molecular shuttles are feasible within a framework that restricts movement and flexibility of the [2]rotaxane axle, and restricts the distance between neighboring molecules, while still allowing for the translational movement of the shuttle. This provides a compelling case for the practical application of MIMs for molecular circuitry.
More information: "A molecular shuttle that operates inside a metal-organic framework" Nature Chemistry, 2015: DOI: 10.1038/nchem.2258
Abstract
A 'molecular shuttle' is an interlocked molecular assembly in which a macrocyclic ring is able to move back and forth between two recognition sites. This large-amplitude translational motion was first characterized in solution in 1991. Since that report, many mechanically interlocked molecules (MIMs) have been designed, synthesized and shown to mimic the complex functions of macroscopic switches and machines. Here, we show that this fundamental concept—the translational motion of a molecular shuttle—can be organized, initiated and made to operate inside a crystalline, solid-state material. A metal–organic framework (MOF) designated UWDM-4 was prepared that contains a rigid linker that is a molecular shuttle. It was demonstrated by variable-temperature 1H-13C cross-polarization/magic-angle spinning (CP/MAS) and 13C 2D exchange correlation spectroscopy (EXSY) solid-state NMR at 21.1 T on a 13C-enriched sample that the macrocyclic ring undergoes rapid shuttling along the rigid axle built between struts of the framework.
Journal information: Nature Chemistry
© 2015 Phys.org