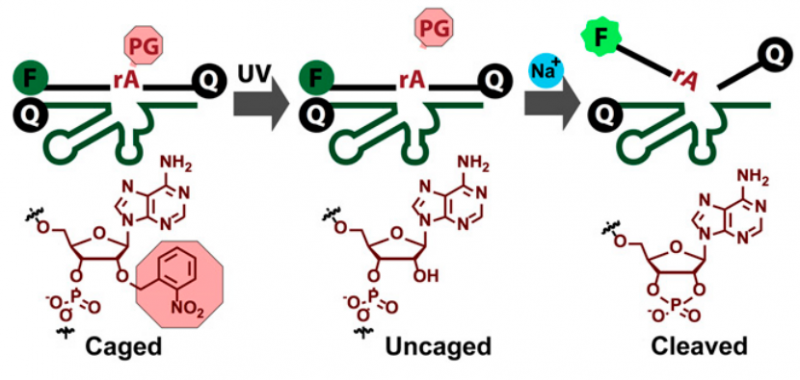
Scheme of the decaging process for the photolabile Na+-specific DNAzyme. Credit: (c) 2015 PNAS, doi: 10.1073/pnas.1420361112
(Phys.org)—Sodium ions are key regulators in cellular processes. The fluids in cells, whether it is water, blood plasma, or nutrients, are regulated by the sodium concentration in cells. If scientists could study sodium ions within a live cell, they would gain important insights into cellular processes including ways to reprogram these processes for biotechnological applications.
However, studying sodium in real-time in living cells has proved difficult. Most biological fluorescence sensors are not selective for sodium, often binding to potassium, or are not feasible in a cellular environment, requiring organic solvents. Other applications cannot provide real-time data. A team from the Departments of Biochemistry, Chemistry, and Materials Science and Engineering at the University of Illinois at Urbana-Champaign has devised a biological fluorescent sensor that is selective for sodium ions and has demonstrated its ability to sense sodium in living cells. Their work was recently published in the Proceedings of the National Academy of Sciences.
The University of Illinois team took advantage of recent developments in developing deoxy-ribozymes, or DNAzymes. DNAzymes are a kind of catalytic DNA that is obtained in the lab using a high throughput selection process. Prior research has demonstrated how DNAzymes can be used as metal ion sensors by designing them to have fluorescent labels that are only "turned on" when the DNAzyme binds the target metal and catalyzes enzymatic reactions. While these studies have demonstrated DNAzymes that can bind monovalent ions, such as Na+ or K+, thus far they have not been selective for sodium over potassium.
The University of Illinois team identified and tested a DNAzyme that is more than 1,000-fold selective for sodium ion over other metals. Furthermore, their DNAzyme can detect sodium concentrations that are within the range typically seen in cells (0.135-50mM), and their detection method is fast enough that real-time studies can be conducted.
DNAzymes can be converted to a fluorescent sensor by placing a fluorophore on one portion of the DNAzyme and a fluorescence quencher on another potion. As long as the fluorophore and the quencher are in contact, only background fluorescence is observed. Once the DNAzyme binds the target metal ion, Na+ in this case, it initiates cleaving a loop of DNA at a particular nucleotide, releasing the substrate portion with the fluorophore. It is separated from the quencher resulting in a fluorescent signal.
DNAzymes are determined by subjecting a library of synthetic DNA candidates to in vitro binding studies using column-based and gel-based selection methods. Potential candidates are then amplified and tested until an optimal candidate is determined. Through this selection and amplification process, this group found a DNAzyme, labeled NaA43, that was selective for Na+.
The next step was to make the fluorescent label. Every DNAzyme has two segments, the substrate and the enzyme strand. For this experiment, as NaA43S and NaA43E are the substrate and enzyme, respectively. The 5' end of NaA43S was labeled with a known fluorophore, and a quencher was placed at its 3'end. An additional quencher was added to the 3' end to ensure a minimal amount of background fluorescence. When Na+ was added, NaA43S was cleaved at the target nucleotide, and the fluorophore was released from quenchers. The result was an increase in fluorescent signal. Furthermore, fluorescence did not significantly change when twenty-two other metal ions were tested.
Finally, the DNAzyme needed to be prepared for cellular insertion and detection. The process of delivering the DNAzyme into the cell could result in premature cleavage, so this team employed "photocaging" to control when the substrate was cleaved. Photocages are photoactive molecules that are placed at the cleavage site to prevent DNA substrate cleavage. When light at a certain wavelength is irradiated at the site, the photo-caged group is released, and then the substrate can be cleaved.
Finally, in order to transport the DNAzyme through the cell membrane and into the cytosol, they used a class of alpha-helical cationic polypeptide that is known to facilitate transportation through the cell membrane. After four-hour incubation into living HeLa cells, NaA43ES was found to be located predominately in the cytosol and did not accumulate in other organelles. The cleavage site was "uncaged" by irradiating the cell with light (365 nm) for thirty minutes. Then they enhanced the sodium levels in the cells. As sodium ions traveled from the extracellular matrix to within the cell, fluorescence measurements increased during this time, demonstrating intracellular Na+ detection in living cells.
This work reports the first use of a DNAzyme to make a real-time, selective sodium ion sensor that can be used in living cells. Since sodium selectivity has been difficult to achieve, and these studies will not only allow for additional studies on cellular activity but may also shed light on ion selectivity, in general.
More information: "In vitro selection of a sodium-specific DNAzyme and its application in intracellular sensing" PNAS, DOI: 10.1073/pnas.1420361112
Abstract
Over the past two decades, enormous progress has been made in designing fluorescent sensors or probes for divalent metal ions. In contrast, the development of fluorescent sensors for monovalent metal ions, such as sodium (Na+), has remained underdeveloped, even though Na+ is one the most abundant metal ions in biological systems and plays a critical role in many biological processes. Here, we report the in vitro selection of the first (to our knowledge) Na+-specific, RNA-cleaving deoxyribozyme (DNAzyme) with a fast catalytic rate [observed rate constant (kobs) ∼0.1 min−1], and the transformation of this DNAzyme into a fluorescent sensor for Na+ by labeling the enzyme strand with a quencher at the 3′ end, and the DNA substrate strand with a fluorophore and a quencher at the 5′ and 3′ ends, respectively. The presence of Na+ catalyzed cleavage of the substrate strand at an internal ribonucleotide adenosine (rA) site, resulting in release of the fluorophore from its quenchers and thus a significant increase in fluorescence signal. The sensor displays a remarkable selectivity (>10,000-fold) for Na+ over competing metal ions and has a detection limit of 135 µM (3.1 ppm). Furthermore, we demonstrate that this DNAzyme-based sensor can readily enter cells with the aid of α-helical cationic polypeptides. Finally, by protecting the cleavage site of the Na+-specific DNAzyme with a photolabile o-nitrobenzyl group, we achieved controlled activation of the sensor after DNAzyme delivery into cells. Together, these results demonstrate that such a DNAzyme-based sensor provides a promising platform for detection and quantification of Na+ in living cells.
Journal information: Proceedings of the National Academy of Sciences
© 2015 Phys.org