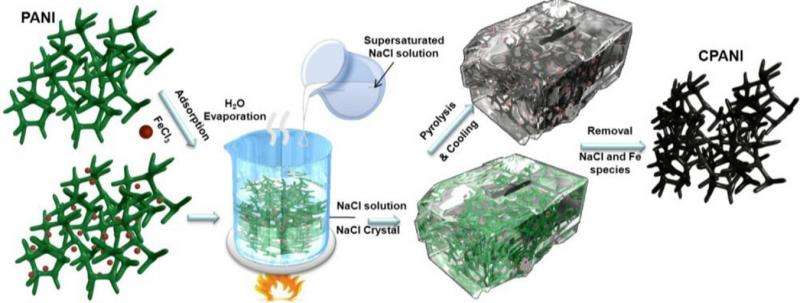
The shape fixing via salt recrystallization method produces porous, carbon-based fuel cell catalysts with a large number of active sites. Credit: Ding, et al. ©2015 American Chemical Society
(Phys.org)—Proton-exchange membrane fuel cells (PEMFCs) are lightweight fuel cells being developed for applications in vehicles and portable electronics. One of the biggest challenges facing their development is the need for expensive platinum-based catalysts. In an effort to lower the cost, scientists are looking for ways to either reduce the amount of platinum required or completely replace the platinum with a less expensive material. But so far, alternative materials have not performed nearly as well as platinum, mainly because they have fewer and less accessible "active sites"—locations where the catalyzed reactions can occur.
To address this challenge, scientists in a new study have developed a way to synthesize materials with a large number of active sites that also ensures that the active sites are accessible to all of the species (electrons, protons, oxygen, and water molecules, etc.) involved in the reactions. They've done this by synthesizing highly porous carbon nanomaterials, in which the pores act as open channels to transport various species to their particular active sites within the carbon framework.
The resulting catalyst, when incorporated into a PEMFC, has a peak power (600 mW/cm2) that is among the best of the non-platinum, non-precious-metal catalysts developed to date. In addition, the researchers explain that the method stands out because it produces the catalysts at a higher yield than any other previous method, in which most products are lost at high temperatures.
The researchers, led by Zidong Wei, Professor of Chemistry at Chongqing University in China, have published their work on the new PEMFC catalyst in a recent issue of the Journal of the American Chemical Society.
The researchers describe the new high-yield method as "shape fixing" because it allows for the construction of carbon nanomaterials with a similar structure and morphology as their polymer precursors. The process of shape-fixing involves pouring a supersaturated sodium chloride (NaCl) solution onto a 3D polyaniline (PANI) carbon-based polymer in a beaker, which results in the water evaporating and NaCl recrystallizing around the PANI until the PANI is fully covered by crystals, almost appearing as if it is buried in a block of ice.
Because the NaCl fully seals the PANI, the researchers explain that the NaCl can be thought of as a nanoreactor. Inside this nanoreactor, the PANI is heated in the processes of pyrolysis and gasification, while various raw materials are added. In the end, the gasification of the various materials in the enclosed space causes the formation of many pores, and the carbonized PANI retains its original 3D shape due to previously being shape-fixed by the NaCl crystal. Further, as the researchers explain, the active sites created in this method are especially highly active.
"The most important results of this work are that the morphology of the polymer is well transferred to the final carbonized materials, more active sites are generated, and it achieves a high production yield," Wei told Phys.org.
The end result is a material with a high density of active sites in easily accessible locations (pores), making it very well-suited as a carbon-based catalyst for proton-exchange membrane fuel cells. The researchers hope to further improve upon the new method in the future, such as by finding new polymer precursors that may perform better than polyaniline.
More information: Wei Ding, et al. "Shape Fixing via Salt Recrystallization: A Morphology-Controlled Approach To Convert Nanostructured Polymer to Carbon Nanomaterial as a Highly Active Catalyst for Oxygen Reduction Reaction." Journal of the American Chemical Society. DOI: 10.1021/jacs.5b00292
Journal information: Journal of the American Chemical Society
© 2015 Phys.org