What do you do when a patient needs a blood transfusion but you don't have their blood type in the blood bank? It's a problem that scientists have been trying to solve for years but haven't been able to find an economic solution - until now.
University of British Columbia chemists and scientists in the Centre for Blood Research have created an enzyme that could potentially solve this problem. The enzyme works by snipping off the sugars, also known as antigens, found in Type A and Type B blood, making it more like Type O. Type O blood is known as the universal donor and can be given to patients of all blood types.
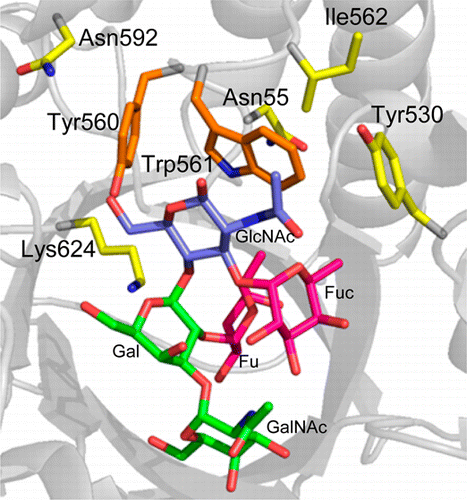
"We produced a mutant enzyme that is very efficient at cutting off the sugars in A and B blood, and is much more proficient at removing the subtypes of the A-antigen that the parent enzyme struggles with," said David Kwan, the lead author of the study and a postdoctoral fellow in the Department of Chemistry.
To create this high-powered enzyme capable of snipping off sugars, researchers used a new technology called directed evolution that involves inserting mutations into the gene that codes for the enzyme, and selecting mutants that are more effective at cutting the antigens. In just five generations, the enzyme became 170 times more effective.
With this enzyme, UBC associate professor Jayachandran Kizhakkedathu and colleagues in the Centre for Blood Research were able to remove the wide majority of the antigens in Type A and B blood. But before it can be used in clinical settings, the enzyme used would need to remove all of the antigens. The immune system is highly sensitive to blood groups and even small amounts of residual antigens could trigger an immune response.
Credit: Wikimedia Commons
"The concept is not new but until now we needed so much of the enzyme to make it work that it was impractical," says Steve Withers, a professor in the Department of Chemistry. "Now I'm confident that we can take this a whole lot further."
The study was published in the Journal of the American Chemical Society.
More information: Toward Efficient Enzymes for the Generation of Universal Blood through Structure-Guided Directed Evolution, J. Am. Chem. Soc., Article ASAP. DOI: 10.1021/ja5116088
Abstract
Blood transfusions are critically important in many medical procedures, but the presence of antigens on red blood cells (RBCs, erythrocytes) means that careful blood-typing must be carried out prior to transfusion to avoid adverse and sometimes fatal reactions following transfusion. Enzymatic removal of the terminal N-acetylgalactosamine or galactose of A- or B-antigens, respectively, yields universal O-type blood, but is inefficient. Starting with the family 98 glycoside hydrolase from Streptococcus pneumoniae SP3-BS71 (Sp3GH98), which cleaves the entire terminal trisaccharide antigenic determinants of both A- and B-antigens from some of the linkages on RBC surface glycans, through several rounds of evolution, we developed variants with vastly improved activity toward some of the linkages that are resistant to cleavage by the wild-type enzyme. The resulting enzyme effects more complete removal of blood group antigens from cell surfaces, demonstrating the potential for engineering enzymes to generate antigen-null blood from donors of various types.
Journal information: Journal of the American Chemical Society
Provided by American Chemical Society