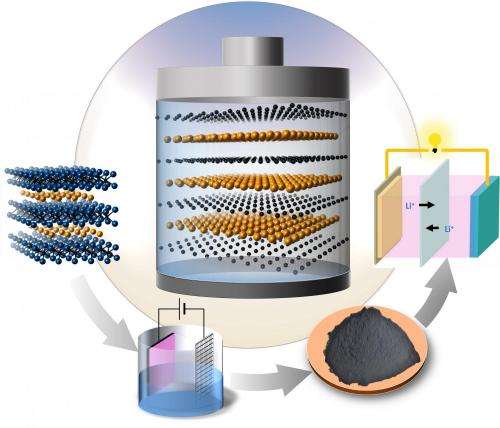
Researchers from Drexel University and Aix-Marseille University have discovered a new high-performance material that could be used for the cathodes of lithium-sulfur batteries. Credit: Drexel University
Not having to constantly charge one's phone or to be able to drive a car for 500 miles without a fill-up or a charge are technical advances most people would appreciate. Drexel researchers, along with colleagues at Aix-Marseille University in France, have discovered a high performance cathode material with great promise for use in next generation lithium-sulfur batteries that would last far longer than what is used today.
In Drexel's Department of Materials Science and Engineering, the nanomaterials research group led by Distinguished University and Trustee Chair Professor Yury Gogotsi, and colleagues at Aix-Marseille University, has created for the first time a 2D carbon/sulfur, C/S, nanolaminate through selectively extracting titanium (Ti) from Ti2SC MAX phase, one of a family of layered ceramics discovered two decades ago by Drexel Materials Distinguished Professor Michel Barsoum. The researchers found that C/S nanolaminates have covalent bonding between C and S and an extremely uniform distribution of sulfur between the atomically thin carbon layers, contributing to their great potential for being used as electrode materials for lithium-sulfur (Li-S) batteries. The international research team published their results, "Synthesis of Carbon/Sulfur Nanolaminates by Electrochemical Extraction of Titanium from Ti2SC" in the prestigious chemistry journal Angewandte Chemie as a VIP paper – only a small percentage of all articles receive this designation recognizing their importance.
Li-S batteries have become one of the hottest topics in the field of energy storage devices due to their high energy density, which is about four times higher than that of Li-ion batteries. One of the major challenges for the practical application of Li-S batteries is to find cathode materials offering long-term stability. Currently, sulfur infiltrated carbon nanomaterials have demonstrated to be the most promising cathode materials for Li-S batteries, in which the uniform distribution of sulfur in carbon matrix and the strong interaction between carbon and sulfur are two important factors that affect the performance. In the C-S nanolaminates synthesized by Gogotsi's group, the sulfur is uniformly distributed in the carbon matrix as atomically thin layers and a strong covalent bonding between carbon and sulfur is observed. As a result, the C-S nanolaminates possess great potential as cathode materials for Li-S batteries. This may have a significant impact on increasing the life-span of next generation batteries.
MAX phases are a class of three-dimensional (3D) layered, ternary carbides and nitrides that are excellent precursors for synthesis of 2D materials through selective extraction due to their ceramic carbide or nitride layers interleaved by atomically thin layers of metal. In 2011, Gogotsi, Barsoum and their PhD student, Michael Naguib, discovered a new class of 2D nanomaterials, called MXenes, by selectively extracting 'A' elements from the MAX phases. MXenes show great promise for applications in the field of energy storage, electronic devices, and composite materials. Later, Gogotsi's student, Maria Lukatskaya, reported the synthesis of carbide-derived carbon by extracting both 'M' and 'A' elements from the MAX phases. Herein, Gogotsi's post-doctoral associate Meng-Qiang Zhao achieved the selective extraction of Ti ('M') from the Ti2SC (MAX), leaving C-S ('AX') nanolaminates via an electrochemical etching, similar to what Lukatskaya used for carbon synthesis but conducted at a lower voltage to prevent oxidation and dissolution of the 'A' element.
Furthermore, the Drexel team also found that it was possible to extract Ti from other MAX phases, such as Ti3AlC2, Ti3SnC2, and Ti2GeC. "We have enough evidence to show that that the electrochemical etching can be a powerful method to selectively extract the "M" elements from the MAX phases, to produce a variety of "AX" layered structures, that cannot be made otherwise," says Zhao. Properties of those are still to be studied.
Gogotsi adds, "We have discovered a great diversity in the 2D materials world. The synthesis of AX structures is another demonstration of the versatility of our synthesis approach. We expect that layered AX structures will demonstrate many useful properties and will find applications beyond battery materials."
Considering that >70 MAX phases are known to exist, not counting solid solutions that easily double or even triple this number, it is not difficult to foresee that the "AX" structures represent a new family of nanostructured materials, much of which will probably be 2D. The various "A" and "X" combinations known render the "AX" structures highly attractive for a number of potential applications, such as electrical energy storage and catalysis, for example. Synthesis of these new "AX" structures, as well as their structural and property characterization, represent a growing area of research, with potentially huge payoffs when useful properties of those materials have been identified.
More information: Angewandte Chemie, onlinelibrary.wiley.com/doi/10 … e.201500110/abstract
Journal information: Angewandte Chemie
Provided by Drexel University