Peering into how rechargeable lithium ion batteries function
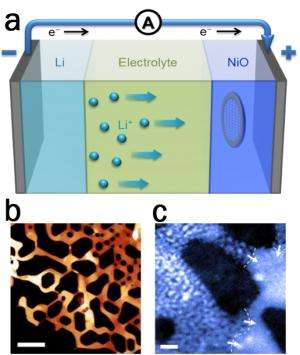
Nanoparticle electrodes in lithium-ion batteries have both near-surface and interior contributions to their redox capacity, each with distinct rate capabilities. Using combined electron microscopy, synchrotron X-ray methods and ab initio calculations, Brookhaven National Laboratory researchers have investigated the lithiation pathways that occur in NiO electrodes. They found that the near-surface electroactive (Ni2+→Ni0) sites saturated very quickly, and then encountered unexpected difficulty in propagating the phase transition into the electrode (referred to as a "shrinking-core" mode).
However, the interior capacity for Ni2+→Ni0 can be accessed efficiently following the nucleation of lithiation fingers, which propagate into the sample bulk, but only after a certain incubation time. Microstructural observations of the transition from a slow shrinking-core mode to a faster lithiation finger mode corroborate with synchrotron characterization of large-format batteries, and can be rationalized by stress effects on transport at high-rate discharge. The finite incubation time of the lithiation fingers sets the intrinsic limitation for the rate capability (and thus the power) of NiO for electrochemical energy storage devices. The present work unravels the link between the nanoscale reaction pathways and the C-rate-dependent capacity loss, and provides guidance for the further design of battery materials that favors high C-rate charging.
Understanding the link between nanoscale reaction pathways and the resulting electric properties of lithium ion batteries can provide considerable information about how to improve the overall design and longevity of these rechargeable batteries.
CFN's Electron Microscopy Facilities were used for atomic scale imaging, spectroscopy, and tomography during the in-situ lithiation process.
More information: "Transitions from Near-Surface to Interior Redox upon Lithiation in Conversion Electrode Materials." Nano Lett. 2015 Feb 11;15(2):1437-44. DOI: 10.1021/nl5049884
Journal information: Nano Letters
Provided by Brookhaven National Laboratory