Metals used in high-tech products face future supply risks
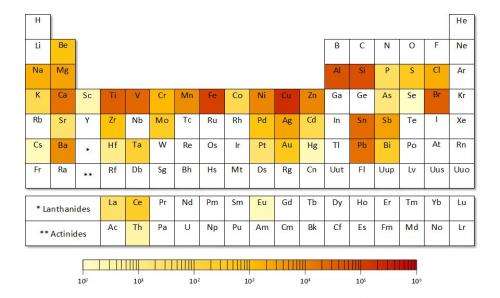
In a new paper, a team of Yale researchers assesses the "criticality" of all 62 metals on the Periodic Table of Elements, providing key insights into which materials might become more difficult to find in the coming decades, which ones will exact the highest environmental costs—and which ones simply cannot be replaced as components of vital technologies.
During the past decade, sporadic shortages of metals needed to create a wide range of high-tech products have inspired attempts to quantify the criticality of these materials, defined by the relative importance of the elements' uses and their global availability.
Many of the metals traditionally used in manufacturing, such as zinc, copper, and aluminum, show no signs of vulnerability. But other metals critical in the production of newer technologies—like smartphones, infrared optics, and medical imaging—may be harder to obtain in coming decades, said Thomas Graedel, the Clifton R. Musser Professor of Industrial Ecology at the Yale School of Forestry & Environmental Studies and lead author of the paper.
The study—which was based on previous research, industry information, and expert interviews—represents the first peer-reviewed assessment of the criticality of all of the planet's metals and metalloids.
"The metals we've been using for a long time probably won't present much of a challenge. We've been using them for a long time because they're pretty abundant and they are generally widespread geographically," Graedel said. "But some metals that have become deployed for technology only in the last 10 or 20 years are available almost entirely as byproducts. You can't mine specifically for them; they often exist in small quantities and are used for specialty purposes. And they don't have any decent substitutes."
These findings illustrate the urgency for new product designs that make it easier to reclaim materials for re-use, Graedel said.
The paper, published in the Proceedings of the National Academy of Sciences, encapsulates the Yale group's five-year assessment of the criticality of the planet's metal resources in the face of rising global demand and the increasing complexity of modern products.
According to the researchers, criticality depends not only on geological abundance. Other important factors include the potential for finding effective alternatives in production processes, the degree to which ore deposits are geopolitically concentrated, the state of mining technology, regulatory oversight, geopolitical initiatives, regional instabilities, and economic policies.
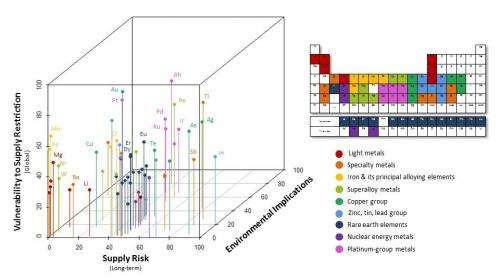
In order to assess the state of all metals, researchers developed a methodology that characterizes criticality in three areas: supply risk, environmental implications, and vulnerability to human-imposed supply restrictions.
They found that supply limits for many metals critical in the emerging electronics sector (including gallium and selenium) are the result of supply risks. The environmental implications of mining and processing present the greatest challenges with platinum-group metals, gold, and mercury. For steel alloying elements (including chromium and niobium) and elements used in high-temperature alloys (tungsten and molybdenum), the greatest vulnerabilities are associated with supply restrictions.
Among the factors contributing to extreme criticality challenges are high geopolitical concentration of primary production (for example, 90 to 95% of the global supply of rare Earth metals comes from China); lack of available substitutes (there is no adequate substitute for indium, which is used in computer and cell phone displays); and political instability (a significant fraction of tantalum, used widely in electronics, comes from the war-ravaged Democratic Republic of the Congo).
The researchers also analyzed how recycling rates have evolved over the years and the degree to which different industries are able to utilize "non-virgin" sources of materials. Some materials, such as lead, are highly recycled because they are typically used in bulk, Graedel said. But the relatively rare materials that have become critical in some modern electronics are far more difficult to recycle because they are used in such miniscule amounts—and can be difficult to extricate from the increasingly complex and compact new technologies.
"I think these results should send a message to product designers to spend more time thinking about what happens after their products are no longer being used," he said. "So much of what makes the recycling of these materials difficult is their design. It seems as if it's time to think a little bit more about the end of these beautiful products."
Journal information: Proceedings of the National Academy of Sciences
Provided by Yale University
















