Trapping carbon dioxide (CO2) emissions from power plants and various industries could play a significant role in reducing greenhouse gas emissions in the future. But current materials that can collect CO2—from smokestacks, for example—have low capacities or require very high temperatures to work. Scientists are making progress toward a more efficient alternative, described in the ACS journal Chemistry of Materials, that could help make carbon capture less energy-intensive.
T. Alan Hatton and colleagues note that although industry and governments are increasingly turning to renewable energy sources such as wind and solar, the world will continue to rely on fossil fuels for the foreseeable future—but at a cost. According to the International Energy Agency, burning fossil fuels emits more than 30 gigatons per year of CO2, a primary greenhouse gas. Some solid systems that aim to capture these emissions, such as zeolites, are sensitive to water in the gas streams. Others, such as clays and metal oxides, have to be heated up to more than 900 degrees Fahrenheit, which requires a lot of energy. Hatton's team wanted to find a way to cut this latter strategy's energy requirements.
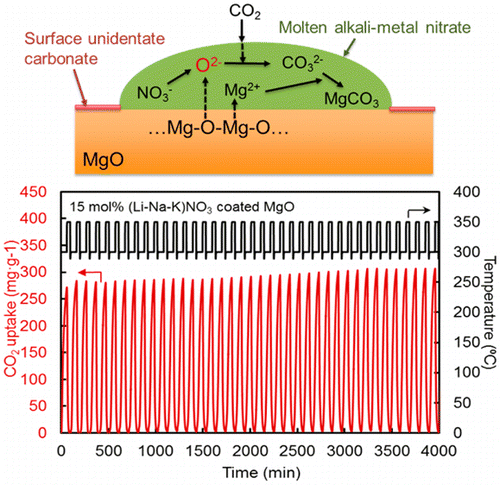
The researchers studied a new class of materials based on magnesium oxide (MgO), which can capture larger quantities of carbon at much lower temperatures than many other substances being investigated. They discovered that coating MgO particles with substances called alkali metal nitrates boosted the amount of CO2 that material could take up by more than 10-fold. The MgO captures a significantly higher amount of CO2 (2-10 times) than other systems for a given volume. This translates into smaller equipment needs and lower plant costs. Additionally, the particles themselves are readily prepared with low-cost materials.
More information:
Alkali Metal Nitrate-Promoted High-Capacity MgO Adsorbents for Regenerable CO2 Capture at Moderate Temperatures, Chem. Mater., Article ASAP
DOI: 10.1021/cm503295g
Abstract
Regenerable high capacity CO2 sorbents are desirable for the establishment of widespread carbon capture and storage (CCS) systems to reduce global CO2 emissions. We report on the marked effects of molten alkali metal nitrates on CO2 uptake by MgO particles and their impact on the development of highly regenerable CO2 adsorbents with high capacity (>10.2 mmol g–1) at moderate temperatures (∼300 °C) under ambient pressure. The molten alkali metal nitrates are shown to prevent the formation of a rigid, CO2-impermeable, unidentate carbonate layer on the surfaces of MgO particles and promote the rapid generation of carbonate ions to allow the high rate of CO2 uptake.
Journal information: Chemistry of Materials
Provided by American Chemical Society