Credit: Illinois Institute of Technology
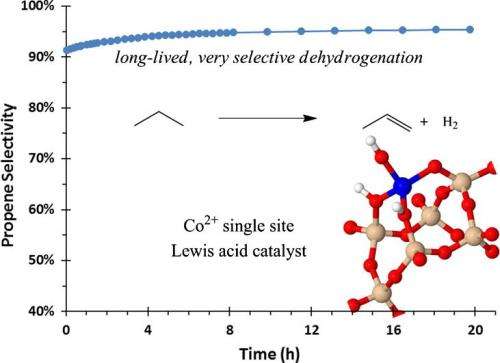
Adam Hock, assistant professor of chemistry at the Illinois Institute of Technology, and colleagues have developed a new cobalt catalyst that selectively transforms propane to propene and hydrogen.
Their research is described in "Selective propane dehydrogenation with single-site CoII on SiO2 by a non-redox mechanism" in the Journal of Catalysis.
Propene, with worldwide sales of $90 billion in 2008, is a crucial product for the petrochemical industry, used in the manufacture of plastics, packaging, and other applications. Catalysts commonly used to transform propane to propene produce propene as well as methane and ethylene. Separating the desired products adds to the cost of the process.
To address this problem, last year Hock and his teammates introduced a new silica-supported single-site zinc catalyst that was more selective for the desired propane to propene transformation, reducing waste, increasing efficiency, and potentially lowering production costs.
The new catalyst is the result of understanding the surface chemistry during the synthesis. This understanding was greatly enhanced by studying the synthesis and operating catalyst using a variety of techniques including the Advanced Photon Source (APS) at Argonne National Laboratory (ANL).
Co-authors on the paper include senior chemists and postdoctoral fellows from ANL, chemistry faculty from Northwestern University, and graduate students from Illinois Tech.
More information: Selective propane dehydrogenation with single-site CoII on SiO2 by a non-redox mechanism, Journal of Catalysis, www.sciencedirect.com/science/ … ii/S0021951714003030
Provided by Illinois Institute of Technology