Biomedical engineer developing nanomaterial for healing broken bones

A new material that triggers stem cells to begin forming bone could enable a more effective treatment for hard-to-heal bone breaks and defects, says a Texas A&M University biomedical engineer who is part of the team developing the biomaterial.
The team's research is detailed in the scientific journal ACS Nano and is supported by the National Science Foundation and the National Institutes of Health. Its findings could change the way medical professionals treat fractured bones that experience difficulty in healing and often require bone graft procedures, says Akhilesh Gaharwar, assistant professor of biomedical engineering at Texas A&M.
The biomaterial, which consists of nano-sized, two-dimensional particles embedded within a gel, stimulates bone growth through a complex signaling mechanism without the use of proteins known as growth factors, Gaharwar explains. Growth factors are used in conventional treatments, but can lead to serious side effects due to the large amounts required to stimulate cells, he says.
"We are trying to overcome these problems by avoiding the use of growth factors as we recapitulate the natural bone-healing process," Gaharwar says. "Our material is a totally different, alternative strategy in which by using minerals we can induce differentiation in stem cells and promote formation of bone-like tissue."
Those minerals, Gaharwar explains, are largely orthosilicic acid, magnesium and lithium – combined in tiny nanosilicate particles that are 100,000 times thinner than a sheet of paper. The ultrathin nanoparticles are embedded in a collagen-based hydrogel, a biodegradable gel used in several biomedical applications because of its compatibility with the body.
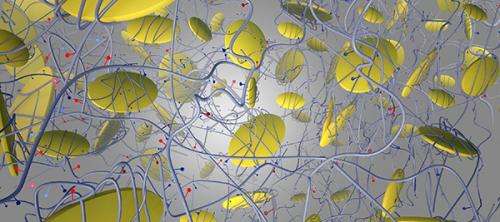
When nanosilicates are incorporated into a gelatin matrix, several physical, chemical and biological properties of the hydrogel are enhanced, Gaharwar explains. For example, the hydrogel can be designed to remain at the injury site for specific durations by controlling the interactions between the nanosilicates and gelatin, Gaharwar adds. This customization, Gaharwar says, can allow the injected hydrogel to enter the defect cavity and help it heal while slowly degrading as it is replaced by natural tissue.
Tests on the mechanical properties of the material are also promising, Gaharwar says. In addition to its ability to be injected at the site of an injury, the material achieves three-to-four times higher stiffness once inside the body, allowing it to be locked in place. This prevents the material from flowing to other parts of the body, thereby avoiding unwanted side effects, Gaharwar says.
The results, Gaharwar says, have been positive, as evidenced by both short-term and long-term indicators of bone growth. Initial tests, he says, show a three-fold increase in alkaline phosphatase activity, a marker for early bone formation (known as osteogenesis). This is confirmation, Gaharwar explains, that the signaling process is indeed "asking" stem cells to differentiate into bone cells. Late markers are also positive, he adds, noting they demonstrate a four-fold increase in the presence of calcium phosphate, a main component of bone.
"The dynamic and bioactive nanocomposite gels we have developed show strong promise in bone tissue engineering applications," Gaharwar says.
As part of future research, Gaharwar plans further investigation into the process by which the nanoplatelets trigger cell differentiation. Additionally, the shear-thinning characteristic of the gel can be used to print three-dimensional tissue structures loaded with cells, he explains. With this in mind, Gaharwar is working with colleagues to build customized, vascularized scaffolds that employ the material and could be surgically inserted at the site of more serious injuries where injection is not an option. The scaffolds, he explains, would allow the injury site to receive blood flow as part of the enhanced healing process initiated by the nanoparticles. It's a system that Gaharwar believes will address some of the challenges associated with engineering complex tissues or organs.
"Based on our strong preliminary studies, we predict that these highly biofunctional particles have immense potential to be used in biomedical applications," he notes.
Journal information: ACS Nano
Provided by Texas A&M University