Slowly evaporating particles refute assumption used in air quality and climate models
Ubiquitous carbon-rich aerosol particles created by emissions from cars, trees, and other sources alter our climate and affect air quality. Until recently, the properties of these aerosols were hard to experimentally characterize, forcing computational models to rely on unsupported assumptions. For several years, scientists at Pacific Northwest National Laboratory have chipped away at these notions. They have provided hard data about viscosity, shape, morphology, volatility, and other fundamental particle properties. Recently, the team tackled how the particles, called secondary organic aerosols (SOAs), evaporate when the relative humidity is high. They found that these aerosols actually evaporate very slowly, sticking around for days.
"Together, our studies of evaporation rates and viscosity provide incontrovertible experimental data that the assumptions invoked to model SOA formation and evolution are fundamentally flawed," said Dr. Alla Zelenyuk, the PNNL chemist who led the study.
Models have persistently and significantly underestimated atmospheric loadings of SOA. Similarly, simulations could not explain how aerosols carry pollutants thousands of miles away from the sources to pristine environments. The assumption that SOAs quickly evaporated got in the way. The SOAs were assumed to be at constant equilibrium with the gas phase through rapid evaporation or condensation and internal mixing. Now, scientists can add aerosols' fundamental properties into the models, opening the door to discover how the most abundant particulates in the atmosphere affect our climate and air quality.
The research group has previously shown that at low relative humidity (RH), SOA particles evaporate much slower than assumed. This led some scientists to assume that, under atmospherically relevant conditions of high RH, these particles would become less viscous and evaporate nearly instantaneously, therefore retaining equilibrium with gas phase.
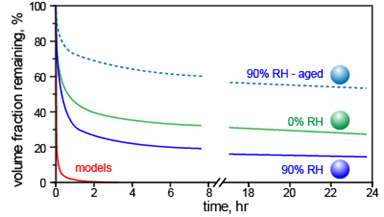
To test the assumption, the team created SOAs from alpha-pinene and limonene, natural aerosol progenitors responsible for the smell of pine trees and lemons. At DOE's EMSL, a national scientific user facility, the team studied the evaporation of these aerosols under RHs ranging from nearly zero to more than 90 percent. Using tools built at EMSL, including SPLAT II, the team showed that in all cases, evaporation rates are orders of magnitude slower than assumed, and way too slow to retain equilibrium with the rapidly changing gas phase. The team showed that the evaporation slowed even more for aged aerosols, which were stored for a day before testing.
"To everyone's surprise, our data clearly showed that while relative humidity affects SOA evaporation kinetics somewhat, the differences are rather small," said Zelenyuk. "At low and high relative humidity, SOAs evaporate too slowly to reach equilibrium with the gas phase." These particles evaporate so slowly that they are best treated as semisolid and non-volatile.
The team is studying how hydrophobic organic molecules, commonly present in the atmosphere, change the aerosols' formation, properties, and behavior. They are focusing on conditions, in which biogenic SOA precursors from trees and other natural sources meet emissions from industrial or other anthropogenic sources.
More information: Wilson J, D Imre, J Beránek, M Shrivastava, and A Zelenyuk. 2015. "Evaporation Kinetics of Laboratory-Generated Secondary Organic Aerosols at Elevated Relative Humidity." Environmental Science & Technology 49(1):243-249. DOI: 10.1021/es505331d
Journal information: Environmental Science & Technology
Provided by Pacific Northwest National Laboratory




















