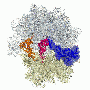Researchers find link in how cells start process necessary for life
Researchers have found an RNA structure-based signal that spans billions of years of evolutionary divergence between different types of cells, according to a study led by researchers at the University of Colorado School of Medicine at the Anschutz Medical Campus and published in the journal Nature.
The finding could alter the basic understanding of how two distinct life forms - bacteria and eukaryotes - begin the process of protein synthesis.
Jeffrey Kieft, PhD, professor of biochemistry and molecular genetics and corresponding author of the article in Nature, said scientists have long thought that the molecular signals that initiate protein synthesis in bacteria and eukaryotes are mutually exclusive. Scientists in Kieft's lab explored whether a structured RNA molecule from a virus that infects eukaryotic cells could function in bacteria. Surprisingly, they found that it could initiate protein syntheses, a process necessary for life.
"What we found bridges billions of years of evolutionary divergence," said Kieft, who is also a Howard Hughes Medical Institute Early Career Scientist. "We wanted to explore whether it was possible to bypass mechanisms that were specific to each domain of life and find a signal capable of operating in both."
Eukaryotes are organisms, such as plants, animals and fungi, whose cells contain a nucleus and are enclosed within membranes, while prokaryotes, such as bacteria, do not contain a nucleus.
In an article that accompanies the study by Kieft and his colleagues, Eric Jan, associate professor in the Department of Biochemistry and Molecular Biology at the University of British Columbia, calls the finding "surprising" and writes that the CU scientists and their colleagues "have shown for the first time that a bona fide signal in an RNA structure promotes protein synthesis in the two domains of life."
Journal information: Nature
Provided by University of Colorado Denver