Understanding cellular ageing
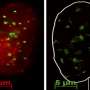
Researchers at the BBSRC-supported Babraham Institute have mapped the physical structure of the nuclear landscape in unprecedented detail to understand changes in genomic interactions occurring in cell senescence and ageing. Their findings have allowed them to reconcile the contradictory observations of two current models of ageing: cellular senescence of connective tissue cells called fibroblasts and cellular models of an accelerated ageing syndrome.
Cellular senescence is an irreversible state of cell cycle arrest and cells enter senescence in response to a variety of stresses. For example, oncogene activation triggers cell senescence as a mechanism to protect against unregulated cell proliferation and the creation of tumours. Cellular senescence is also thought to have a role in normal developmental processes and hence in ageing.
In the first model, cellular senescence triggers large-scale spatial rearrangements of chromatin and the formation of dense nuclear domains called SAHF (senescence associated heterochromatic foci, seen as blue spots in the image above). Chromatin is the complex of DNA and proteins that forms the chromosomes in the nucleus. The second model uses fibroblast cells from people with a syndrome causing accelerated ageing (Hutchinson-Gilford progeria syndrome, HGPS) and these cells show reduced compaction of chromatin and do not show the creation of SAHF domains.
The Babraham Institute researchers measured the frequency of genome interactions occurring throughout the whole genome in senescent fibroblasts and compared them to studies on HGPS cells. This approach brought together scientists from two of the Institute's core research programmes: epigenetics and nuclear dynamics. Unexpectedly, they found that SAHF regions, thought to be highly condensed and structured, show a dramatic loss of local interconnectivity and internal structure in senescence chromatin and that this effect was also seen in the genomes from HGPS cells. Looking in detail at the genomic events occurring during cell senescence allowed the researchers to resolve SAHF formation into two stages: 1) changes in local connectivity in the genome, similar to those found in HGPS and 2) the senescence-specific clustering of these regions, creating the SAHF domains.
Dr Tamir Chandra, lead author and postdoctoral researcher based at both the Babraham Institute and Wellcome Trust Sanger Institute, said: "The seemingly opposite changes in chromatin behaviour between cell senescence and cells from HGPS patients have been an obstacle to understanding their contribution to ageing. Using physical interaction mapping, a direct measure of the genome architecture, our study suggests that the chromatin does initially change in a similar way in cell senescence and HGPS. We can now focus our studies on these early events common to both model systems."
Professor Wolf Reik, Group Leader and Associate Director at the Babraham Institute and Associate Faculty at the Wellcome Trust Sanger Institute said: "There are probably important aspects of ageing which are regulated or influenced by epigenetic mechanisms such as chromatin compaction. It is therefore important to understand dynamic changes of epigenetic marks during ageing, how they come about, and what impact they have on altered cell function later in life".
The research, published in the journal Cell Reports, provides a common model of cellular ageing supported by both the study of senescence and progeria. Having a better understanding of the biological events contributing to ageing will result in benefits to health, wellbeing and independence in later life. This research was funded by support from the BBSRC and the Wellcome Trust.
More information: Chandra, Ewels et al. (2015). Global reorganisation of the nuclear landscape in senescent cells. Cell Reports, 2015.
Journal information: Cell Reports