Nanotechnology against malaria parasites
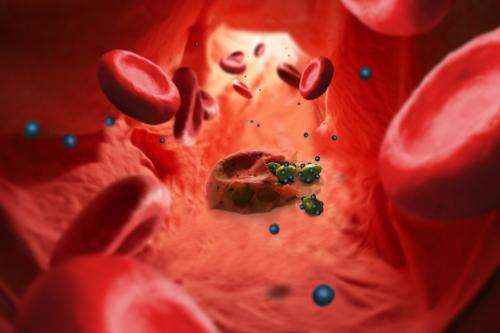
Malaria parasites invade human red blood cells, they then disrupt them and infect others. Researchers at the University of Basel and the Swiss Tropical and Public Health Institute have now developed so-called nanomimics of host cell membranes that trick the parasites. This could lead to novel treatment and vaccination strategies in the fight against malaria and other infectious diseases. Their research results have been published in the scientific journal ACS Nano.
For many infectious diseases no vaccine currently exists. In addition, resistance against currently used drugs is spreading rapidly. To fight these diseases, innovative strategies using new mechanisms of action are needed. The malaria parasite Plasmodium falciparum that is transmitted by the Anopheles mosquito is such an example. Malaria is still responsible for more than 600,000 deaths annually, especially affecting children in Africa (WHO, 2012).
Artificial bubbles with receptors
Malaria parasites normally invade human red blood cells in which they hide and reproduce. They then make the host cell burst and infect new cells. Using nanomimics, this cycle can now be effectively disrupted: The egressing parasites now bind to the nanomimics instead of the red blood cells.
Researchers of groups led by Prof. Wolfgang Meier, Prof. Cornelia Palivan (both at the University of Basel) and Prof. Hans-Peter Beck (Swiss TPH) have successfully designed and tested host cell nanomimics. For this, they developed a simple procedure to produce polymer vesicles – small artificial bubbles – with host cell receptors on the surface. The preparation of such polymer vesicles with water-soluble host receptors was done by using a mixture of two different block copolymers. In aqueous solution, the nanomimics spontaneously form by self-assembly.
Blocking parasites efficiently
Usually, the malaria parasites destroy their host cells after 48 hours and then infect new red blood cells. At this stage, they have to bind specific host cell receptors. Nanomimics are now able to bind the egressing parasites, thus blocking the invasion of new cells. The parasites are no longer able to invade host cells, however, they are fully accessible to the immune system.
The researchers examined the interaction of nanomimics with malaria parasites in detail by using fluorescence and electron microscopy. A large number of nanomimics were able to bind to the parasites and the reduction of infection through the nanomimics was 100-fold higher when compared to a soluble form of the host cell receptors. In other words: In order to block all parasites, a 100 times higher concentration of soluble host cell receptors is needed, than when the receptors are presented on the surface of nanomimics.
"Our results could lead to new alternative treatment and vaccines strategies in the future", says Adrian Najer first-author of the study. Since many other pathogens use the same host cell receptor for invasion, the nanomimics might also be used against other infectious diseases. The research project was funded by the Swiss National Science Foundation and the NCCR "Molecular Systems Engineering".
More information: Adrian Najer, Dalin Wu, Andrej Bieri, Françoise Brand, Cornelia G. Palivan, Hans-Peter Beck, and Wolfgang Meier. "Nanomimics of Host Cell Membranes Block Invasion and Expose Invasive Malaria Parasites." ACS Nano, Publication Date (Web): November 29, 2014 | DOI: 10.1021/nn5054206
Journal information: ACS Nano
Provided by University of Basel