Researchers turn gold nanoclusters into 12-sided dodecahedron crystals
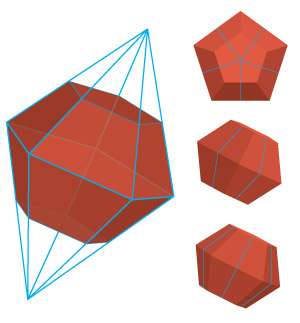
A*STAR researchers have devised a way to destabilize gold nanoclusters so that they form tiny atomic nuclei that then grow together into perfectly proportioned, 12-sided dodecahedron crystals. These unique polyhedra have energy-rich surfaces that can boost the catalytic efficiency of important chemical reactions and serve as potential adsorption sites for targeted sensor devices.
Typically, gold nanoclusters are prepared by chemically reducing a gold–sulfur precursor in the presence of an organic stabilizing agent. This procedure creates a symmetric core of gold atoms protected by a thin layer of surface groups known as thiolates. Researchers have developed many techniques for varying the size of the nanoclusters to tune their chemical and physical properties. But destabilizing gold-thiolate bonds to enable further transformations into polyhedral crystals has proved more challenging.
To tackle this issue, an interdisciplinary team led by Yong-Wei Zhang from the Institute of High Performance Computing and Ming-Yong Han from the Institute of Materials Research and Engineering at A*STAR in Singapore investigated strategies to destabilize gold clusters by oxidizing the surface-protecting thiolates. While promising, this approach has its risks: previous attempts using ozone destabilization agents produced uncontrolled aggregation of gold atoms into macroscopic precipitates.
The researchers examined if switching to a milder hydrogen peroxide destabilization agent would give more favorable results. They first synthesized a solution of 25-atom gold clusters stabilized by outer layers of bovine serum albumin (BSA). When hydrogen peroxide was added to the mixture, the team's mass spectrometry instruments showed that covalent gold–sulfur bonds slowly ruptured.
This peroxide-based destabilization initially produced smaller 11-atom gold clusters. But after sitting for nearly a week at room temperature, these clusters transformed into remarkable dodecahedron shapes (see image).
High-resolution scanning electron microscopy revealed that every facet on the dodecahedra had identical crystallographic orientations—a rare distribution of gold atoms known as a [110] facet. Density functional theory calculations initiated by co-author Guijian Guan showed that these unusual structures arise when amino acids liberated from BSA during the destabilization reaction attach to the nanoparticles and promote growth in every crystal direction except the [110] orientation.
Guan explains that because [110] facets have the highest surface energies among standard gold facets, they present strong attractive forces to incoming molecules—a phenomenon that improves the catalytic capacity due to a stronger binding affinity to target molecules. "For example, we observed a four-fold enhancement in catalytic ability for our dodecahedra compared to gold nanoparticles during the reduction of 4-nitrophenol to 4-aminophenol," he notes.
More information: Guan, G., Liu, S., Cai, Y., Low, M., Bharathi, M. S. et al. "Destabilization of gold clusters for controlled nanosynthesis: From clusters to polyhedra." Advanced Materials 26, 3427–3432 (2014). dx.doi.org/10.1002/adma.201306167
Journal information: Advanced Materials



















