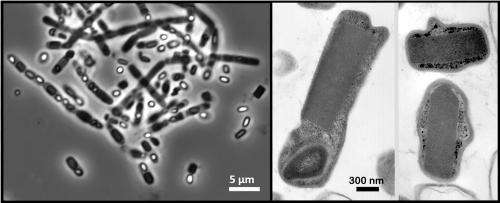
At left, this phase-contrast micrograph image shows a cluster of rod-shaped bacterial cells (Bacillus thuringiensis, or "Bt") suspended in pure water. The dark rectangular shapes inside the cells correspond to naturally occurring crystals within the cells, while the bright white oval shapes correspond to spores. The transmission electron micrograph images at right show enlarged views of the rectangular crystals contained in the cells. (10.1073/pnas.1413456111)
(Phys.org) —Scientists have for the first time mapped the atomic structure of a protein within a living cell. The technique, which peered into cells with an X-ray laser, could allow scientists to explore some components of living cells as never before.
The research, published Aug. 18 in Proceedings of the National Academy of Sciences, was conducted at the Department of Energy's SLAC National Accelerator Laboratory.
"This is a new way to look inside cells," said David S. Eisenberg, a biochemistry professor at University of California, Los Angeles, and Howard Hughes Medical Institute investigator.
"There are a lot of semi-ordered materials in cells where an X-ray laser could provide powerful information," Eisenberg added. They include arrays in white blood cells that help to fight parasites and infections, insulin-containing structures in the pancreas and structures that break fatty acids and other molecules into smaller units to release energy.
In the experiment at SLAC's Linac Coherent Light Source X-ray laser, a DOE Office of Science User Facility, researchers probed a soil-dwelling bacterium, Bacillus thuringiensis or Bt, that is commonly used as a natural insecticide. Strains of this bacterium produce microscopic protein crystals and spores that kill insects. Normally scientists need to find ways to crystallize proteins in order to get their structures – typically a time-consuming, hit-and-miss process – but these naturally occurring crystals eliminated that step.
A liquid solution containing the living cells was jetted into the path of the ultrabright LCLS X-ray laser pulses. When a laser pulse struck a crystal, it created a pattern of diffracted X-ray light. More than 30,000 of these patterns were combined and analyzed by sophisticated software to reproduce the detailed 3-D structure of the protein.
Three scenarios suggesting how the integrity of Bacillus thuringiensis (Bt) cells studied at the Linac Coherent Light Source X-ray laser might vary at the moment they are struck by X-rays. The horizontal arrow depicts the flow of the cell samples from a liquid jet to waste collection. The left, middle, and right columns depict three different time points along the liquid jet's stream. Depending on the rate of cell rupture and the flow rate of the jet, the crystals may arrive at the interaction point either (1) inside intact cells, (2) inside ruptured ("lysed") cells, or (3) outside of ruptured cells. (10.1073/pnas.1413456111)
Many of the bacterial cells likely ruptured and spewed their crystal contents as they flew at high speed toward the X-rays. But because it took just thousandths of a second for the cells to reach the X-ray pulses, it's very likely that many of the X-ray images showed protein crystals that were still inside the cells, the researchers concluded.
Importantly, Eisenberg said, "The rest of the cell contents don't obscure the results."
In addition, the 3-D structure of proteins obtained from the crystals in living bacteria cells was essentially identical to that obtained through other methods. Earlier studies had already shown that LCLS can be used to study smaller, easier-to-produce crystals than traditional X-ray sources require, although it typically requires a far larger volume of crystals to achieve atomic-scale resolution.
In an LCLS study published in 2012, a separate team of researchers used protein crystals grown inside live insect cells to study a potential weak spot in a parasite responsible for a disease called African sleeping sickness. But in that experiment they extracted the crystals rather than attempting to study them inside cells.
Eisenberg said possible next steps include improving the technique by developing new sample-delivery methods that are gentler to the cells' structure, and producing faster X-ray pulse rates that capture more images and yield even better results.
"I think this whole area of science is going to continue growing," he said.
More information: Michael R. Sawaya, Duilio Cascio, Mari Gingeri et al., PNAS, 18 August 2014 (10.1073/pnas.1413456111)
Journal information: Proceedings of the National Academy of Sciences
Provided by SLAC National Accelerator Laboratory