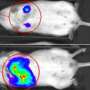
Driving cancer cells to suicide
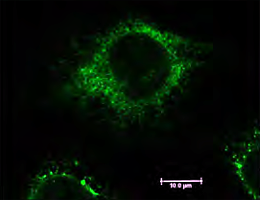
Ludwig Maximilian University of Munich researchers report that a new class of chemical compounds makes cancer cells more sensitive to chemotherapeutic drugs. They have also pinpointed the relevant target enzyme, thus identifying a new target for anti-tumor agents.
Researchers led by LMU's Professor Angelika Vollmar and Professor Stephan Sieber of the Technische Universität München have identified a class of chemicals that represent a potential new weapon in the fight against malignant tumors. The compound is itself non-toxic, but it stimulates the killing of rapidly dividing cells by chemotherapeutic drugs. This sensitizing effect means that the latter can be used in lower doses, which makes it less likely that the target cells will become resistant to their lethal effect. The work was carried out by an interdisciplinary collaboration made up of scientists from LMU, TUM and the Saarland University in Saarbrücken, and the results appear in the latest issue of the journal "Angewandte Chemie – International Edition".
Chemotherapy of malignant tumors is complicated by the fact that, over time, rapidly dividing cancer cells tend to become resistant to the drugs used. "One way to avoid this is to administer the agent in conjunction with an otherwise innocuous compounds that makes cells more vulnerable to its deleterious effects, and induces them to undergo programmed cell death," says Angelika Vollmar, Professor of Pharmaceutical Biology at LMU.
The collaborative venture has led to the discovery of new class of chemical compounds, referred to as T8, which specifically sensitizes cells to the effect of the anti-cancer drug etoposide, which inhibits the growth of tumor cells by inducing the formation of breaks in the DNA. "The interdisciplinary approach and the close cooperation between chemists and biologists made a crucial contribution to the success of our project," says Vollmar. The scientists have also identified protein disulfide isomerase (PDI) as the target of the new agents. PDI is an enzyme that modifies the spatial conformation, and thus the functional state, of proteins involved in a wide variety of cellular functions.
A major advantage of the new compound is that it is intrinsically non-toxic. Moreover, its functional impact on its target enzyme is reversible. Only when it is administered together with a chemotherapeutic agent do its effects on cellmetabolism become manifest. "The combination of a sub-toxic concentration of etoposide with the new T8 compounds makes cells more susceptible to programmed cell death," says Vollmar.
The researchers elucidated the new compound's mode of action in a series of cell biological and biochemical experiments. "Our studies show that T8 is a very promising lead compound, as it is capable of exercising a chemosensitizing effect on diverse types of cancer cells. The drug has been tested on a variety of different tumor cells including leukemia, pancreatic and breast cancer cell lines. "In the next phase of the project, this new class of chemosensitizers will be optimized and tested in a variety of in-vivo animal models, and the compounds will be used to probe the functional significance of PDI as a drug target for tumor therapy." The work was supported by grants from the Wilhelm Sander Foundation and the DFG.
More information: Eirich, J., Braig, S., Schyschka, L., Servatius, P., Hoffmann, J., Hecht, S., Fulda, S., Zahler, S., Antes, I., Kazmaier, U., Sieber, S. A. and Vollmar, A. M. (2014), "Eine niedermolekulare Verbindung inhibiert die Proteindisulfidisomerase und sensibilisiert Krebszellen für Chemotherapie." Angew. Chem.. doi: 10.1002/ange.201406577
Journal information: Angewandte Chemie
Provided by Ludwig Maximilian University of Munich